Lineus acutifrons Southern, 1913
|
publication ID |
https://doi.org/ 10.1080/00222933.2010.504895 |
|
persistent identifier |
https://treatment.plazi.org/id/03FC878F-4D6C-A806-FE25-622352E3FBA3 |
|
treatment provided by |
Felipe |
|
scientific name |
Lineus acutifrons Southern, 1913 |
| status |
|
Lineus acutifrons Southern, 1913 View in CoL
Lineus acutifrons Southern 1913: 7–9 View in CoL , pl. I, fig. 1A–D; Gibson 1982: 84, fig. 20A; Gibson and Knight-Jones 1990: 153, fig. 5.3G; Gibson 1994: 88, fig. 21A.
Description
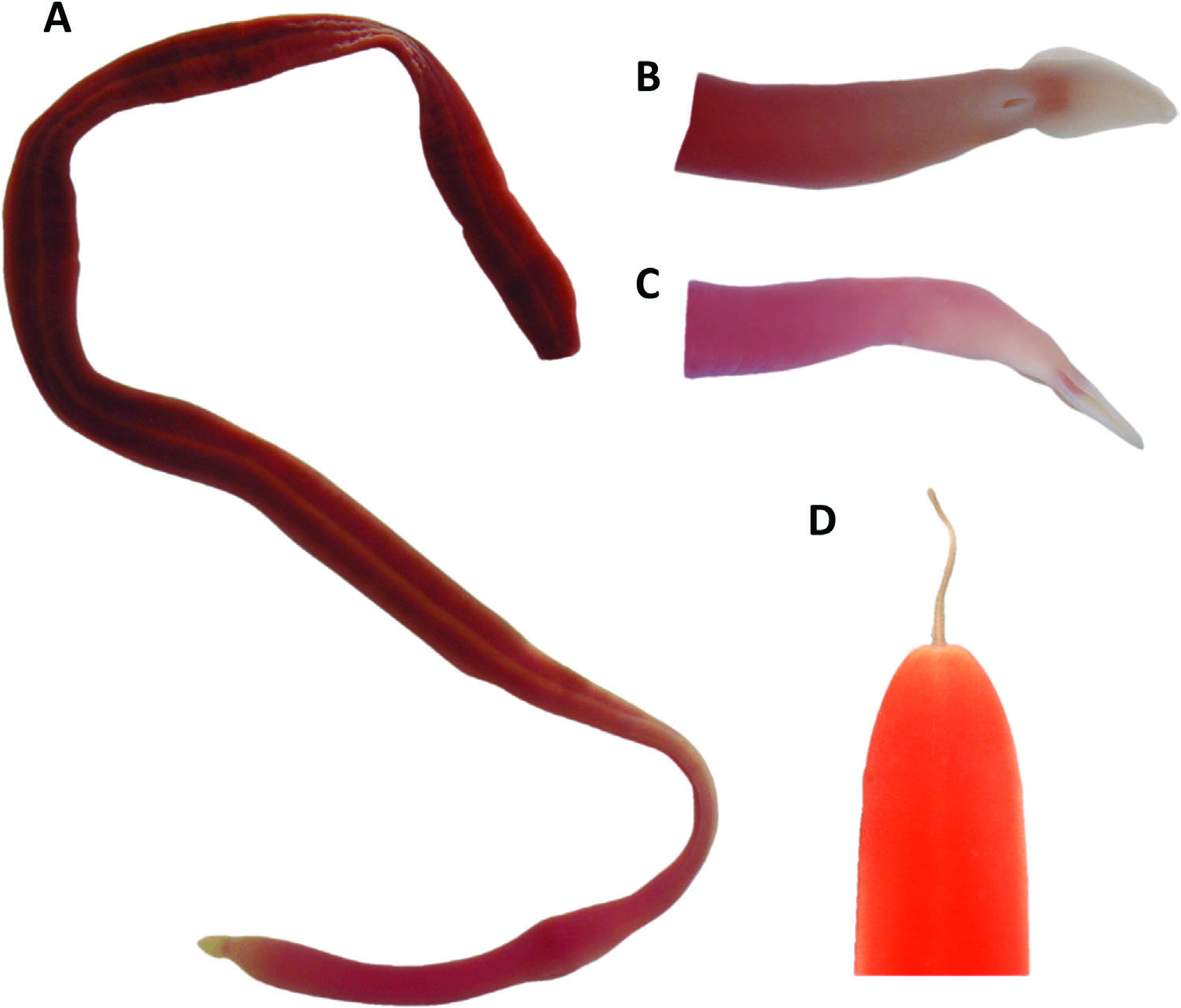
External features. Live specimens measured up to 250 mm long and 5 mm maximum diameter before and after being anaesthetized. Anterior body region rounded in cross-section, attened towards mid to posterior parts ( Figure 2A View Figure 2 ); this body regionalization more apparent in larger worms. Smaller worms cylindrical throughout body length. Head ovate and acutely pointed in front, marked off from body by well-defined rounded constriction in front of mouth ( Figure 2B View Figure 2 ). Long, wide, deep pair of horizontal cephalic slits present, posteriorly expanded to form deep bays ( Figure 2C View Figure 2 ). Eyes absent. Pale caudal cirrus is present ( Figure 2D View Figure 2 ). Body dark or brick red, fades to pink in anterior region, until head, which is pink to white. No differences in colouration between dorsal and ventral surfaces.
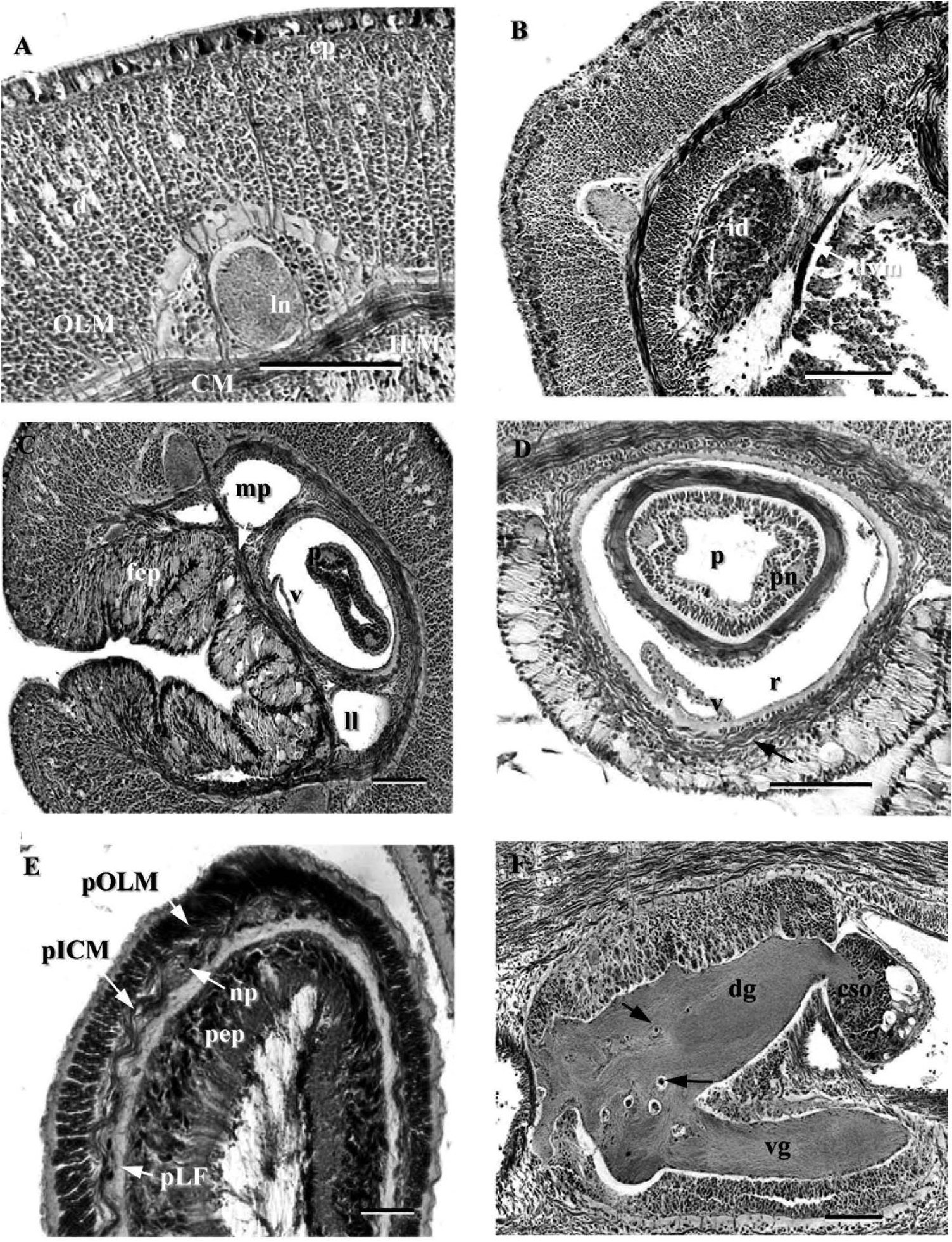
Body wall and musculature. Epidermis ciliated and glandular 20–25 µm thick, possesses typical heteronemertean arrangement. Thin subepidermal muscle layer, four or five fibres, 6 µm thick. Dermal gland cells form no distinctive layer, basophilic gland cells interspersed between outer longitudinal muscle fibres (36–40 µm thick). Body wall muscles well-developed throughout the post-cerebral regions consist of outer longitudinal muscle, circular muscle and inner longitudinal muscle layers, each respectively 180–200, 45–50 and 10–12 µm thick; thin connective tissue layer separates outer longitudinal muscle from circular muscle. The former is composed of large muscle fibre bundles, penetrated regularly by radial connective tissue and muscle fibre strands. These radially oriented fibres penetrate the ganglionic zone of lateral nerve cords ( Figure 3A View Figure 3 ). Dermal gland cells distributed in homogeneous layer in the intestinal region. Thickness of three muscle layers, outer longitudinal muscle, circular muscle and inner longitudinal muscle, is 100–120, 30–35, and 40–45 µm, respectively ( Figure 3B View Figure 3 ).
Rhynchodaeum and cephalic lacuna surrounded by a coat of longitudinal and circular muscle in the pre-cerebral region. Radial and diagonal fibres run through the head, intermingled with some basophilic glandular areas and several anteriorly directed nerves. Typical heteronemertean body wall morphology becomes apparent at the cerebral ganglia, the three body wall muscle layers distinguishable at the opening of the mouth about midway.
Well-developed horizontal muscle plate between rhynchocoel and mouth epithelium, 10–20 µm thickness ( Figure 3C View Figure 3 ). At the intestinal region, dorsoventral fibres from the body wall run between intestinal diverticula ( Figure 3B View Figure 3 ).
Proboscis apparatus. Proboscis pore ventral, close to head, tip continuous with the rhynchodaeum. Rhynchocoel extends from the proboscis insertion to the posterior end of the body. Its wall consists of two muscle layers; longitudinal layer one or two fibres thick and thicker circular muscle layer. Rhynchocoel circular muscle layer is thicker in the intestinal region than foregut region. At the villus, rhynchocoel circular musculature interweaves with foregut musculature ventral and laterally ( Figure 3D View Figure 3 ).
Proboscis relatively slender, approximately one-fifth to one-sixth of the body’s diameter. Thick glandular acidophilic epithelium, underlain by connective tissue. Below this connective tissue, some longitudinal muscle fibres irregularly interspersed but without forming a distinct layer. A pair of proboscis nerves distinguishable at the anterior part and are the origin of posterior neural plexus. This palaeotype proboscis formed also by very thick circular muscle layer (15–20 µm), outer longitudinal muscle stratum, connective tissue and outer epithelium. No indication of muscle crosses has been observed ( Figure 3D, E View Figure 3 ).
Alimentary canal. Mouth opens ventrally near the brain, just behind cephalic constriction ( Figure 2B View Figure 2 ). Beneath the thick (80–160 µm) ciliated glandular folded epithelium, a vascular plexus and two subepithelial nerves can be distinguished ( Figure 3C View Figure 3 ). These nerves are not visible at posterior foregut. Intestine well developed bears long lateral diverticula ( Figure 3B View Figure 3 ).
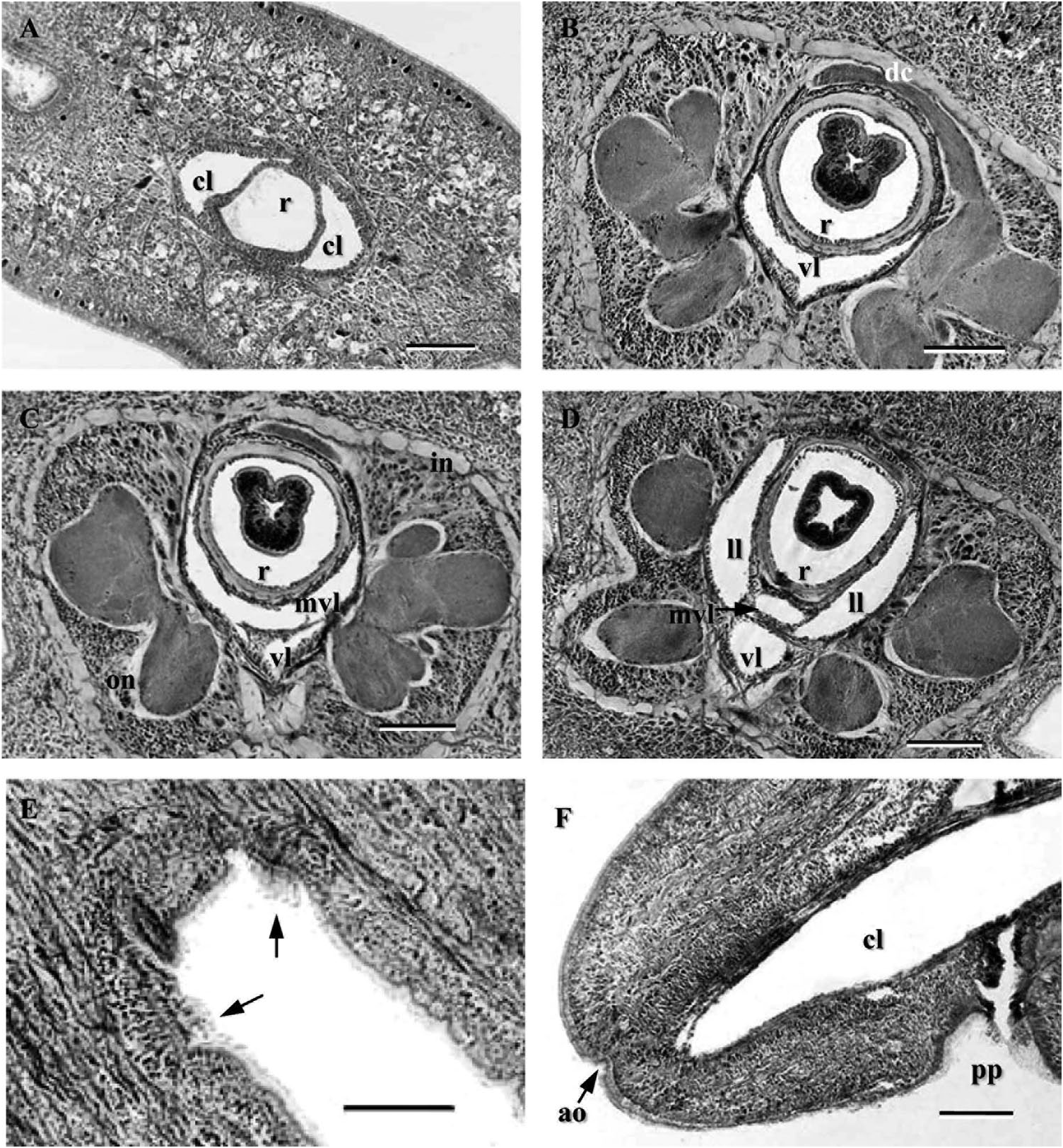
Blood system. Single large median blood lacuna at the tip of head, divided into two spacious lateral cephalic lacunae by a dorsoventral extension of rhynchodaeum ( Figure 4A View Figure 4 ). At cerebral ganglia, these two lacunae extend ventrally and meet below rhynchocoel to form “U”-shaped ventral lacuna ( Figure 4B View Figure 4 ); this lacuna is divided by connective tissue strands into medial ventral lacunae ( Figure 4C View Figure 4 ). Posteriorly, the latter lacuna is again divided in two; at this level, two lateral lacunae and two mid-ventral lacunae are present ( Figure 4D View Figure 4 ). An upper mid-ventral lacuna penetrates rhynchocoel forming a villus, which runs posterior to the end of foregut, and is the origin of a medial blood vessel ( Figure 3D View Figure 3 ). At the mouth, lateral lacunae spread through lateral regions forming foregut vascular plexus as described for many pilidiophorans. Three longitudinal blood vessels run along most of body length with no transverse connective between them.
Nervous system. Well-developed brain, both inner and outer neurilemma evident, outer layer thicker ( Figure 4B–D View Figure 4 ). Dorsal and ventral lobes similar in size. Dorsal commissure long and slender (20–30 µm wide), ventral commissure comparatively short and thick (40 µm wide). As in other pilidiophorans, dorsal ganglia bifurcates posteriorly into the cerebral sensory organ ( Figure 3F View Figure 3 ). Two thick nerves from the ventral ganglia innervate the mouth. Ganglia lacks neurochord cells. Putative gregarine parasites observed in the cerebral ganglion tissue ( Figure 3F View Figure 3 ). Lateral nerve cords emerge from ventral brain lobes, and inner and outer persist. No neurochord cells were observed within the lateral nerve cords.
Sense organs. Head bears a pair of long, wide and deep horizontal cephalic slits as in Lineidae (sensu Friedrich 1960; Gibson 1985); in living specimens, upper and lower margins of the slit do not meet medially, slit is open ( Figure 2C View Figure 2 ). Ciliated cerebral canals emerge from posterior dorsal wall of cephalic bays, which is more glandular and has longer cilia ( Figure 4E View Figure 4 ). Cerebral sensory organs situated behind dorsal brain lobes, inside lateral blood lacunae ( Figure 3F View Figure 3 ). Single apical sense organ observed as small invagination at very tip of head in longitudinal sections ( Figure 4F View Figure 4 ). No pigment cup ocelli observed.
Excretory system. No traces of nephridial structures observed.
Reproductive system. No mature specimens found.
Habitat and behaviour
Lineus acutifrons lives in sandy beaches, in the lower part of the intertidal slope, buried in the sediment. From the 18 beaches surveyed, the species was only collected from four that have a at dissipative area, with sediments from fine to medium sands ( Junoy et al. 2005). Density in these beaches was very low, with a maximum of one specimen per 30 m 2. The macroinfaunal community of these beaches consisted of typical psammophilous species dominated by crustaceans and polychaetes. The most frequent and abundant species were the amphipod Pontocrates arenarius (Bate, 1858) , the mysid Gastrosaccus roscoffensis Bacescu, 1970 and the spionid polychaetes Scolelepis mesnili (Bellan and Lagardère, 1971) and Scolelepis squamata Müller, 1806 . Other accompanying species were the isopods Eurydice naylori Jones and Pierpoint, 1997 and Eurydice affinis Hansen, 1905 , the amphipod Haustorius arenarius (Slabber, 1769) and the polychaete Nephtys cirrosa Ehlers 1868 . These species were offered to L. acutifrons in individual containers, but no proboscis eversion was observed and no prey were consumed. This nemertean is the largest macrofaunal species of the beaches and presumably eats larger polychaetes present in the habitat, such as S. squamata and N. cirrosa .
Specimens maintained in the laboratory were surrounded by a mucus sheath with sand grains; however, this cover was not visible at collecting times. A singular behaviour was observed in Petri dishes whereby the posterior end waved around while the anterior part was still, which is the opposite of that seen in other Pilidiophora.
Phylogenetic analysis
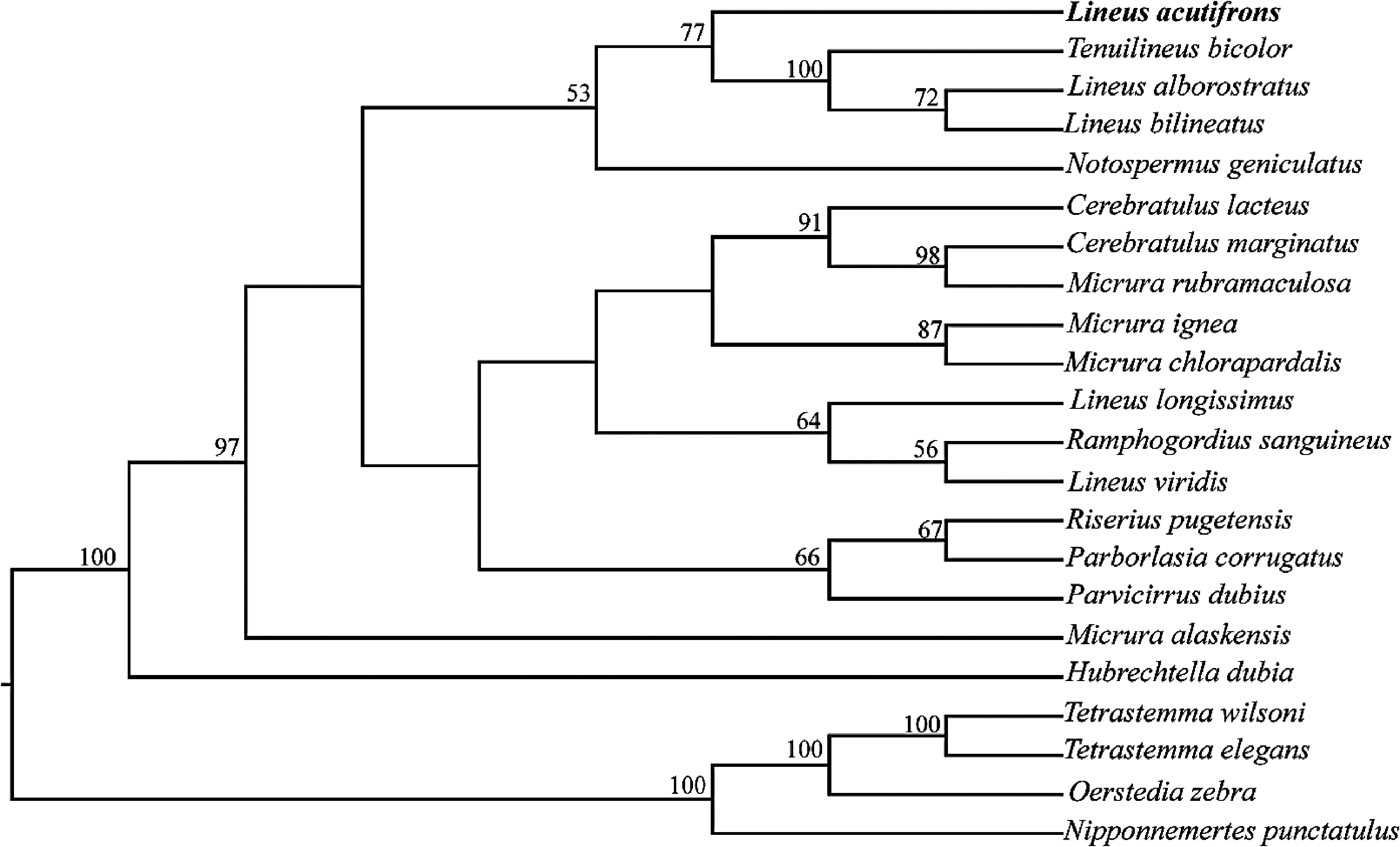
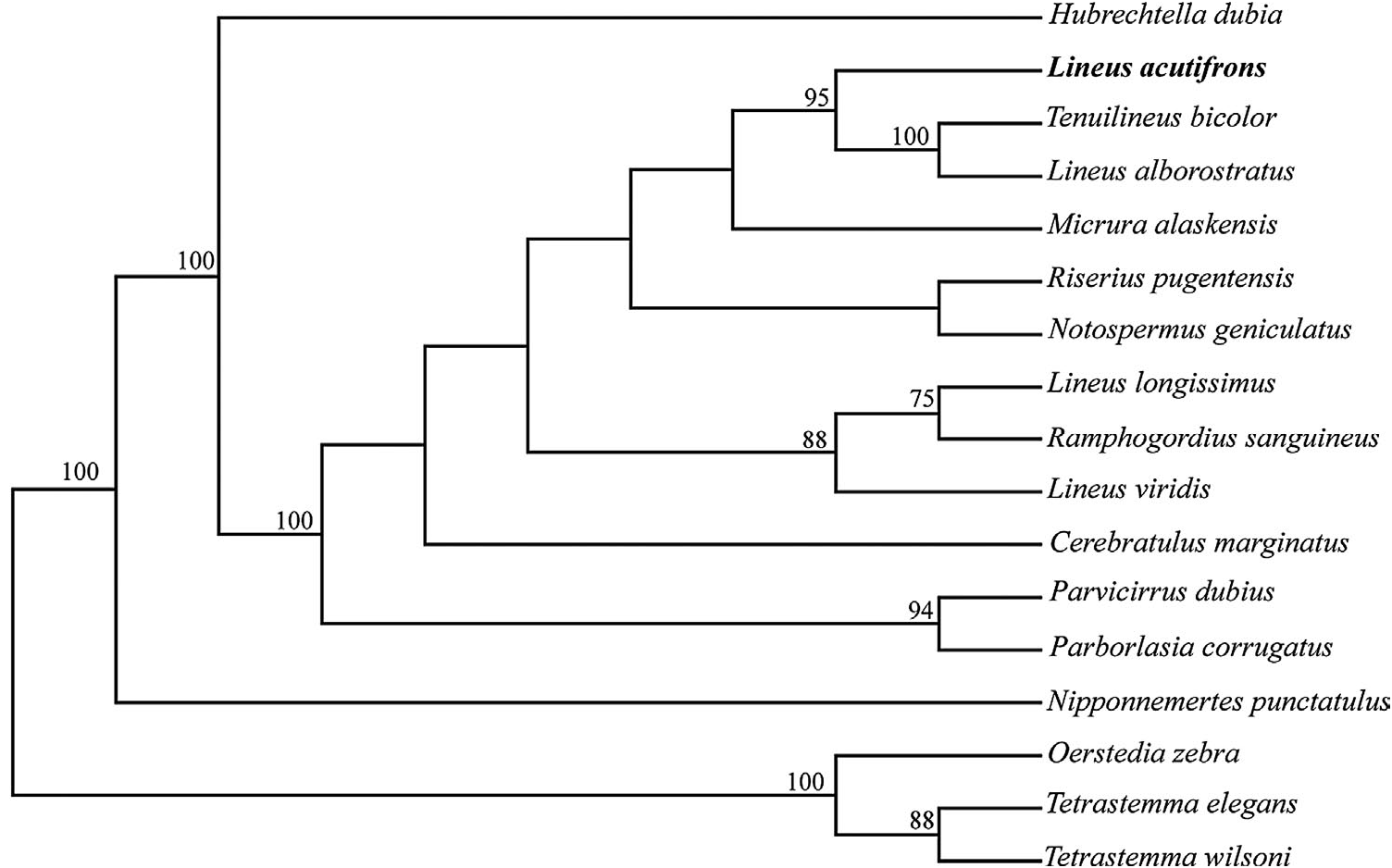
The phylogenetic hypothesis obtained using exclusively 28S data supports a relationship between Tenuilineus bicolor , Lineus alborostratus and Lineus bilineatus with bootstrap support of 77% ( Figure 5 View Figure 5 ). The individual phylogenies from CO1 and 28S, and the combined phylogeny of both genes demonstrate similar results: Lineus acutifrons is in a clade with other members of the Lineidae family, which was supported by high bootstrap values. In both the CO1 and combined phylogenies, L. acutifrons is a sister species to L. alborostratus and T. bicolor with 95% bootstrap support ( Figure 6 View Figure 6 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lineus acutifrons Southern, 1913
| Puerta, Patricia, Andrade, Sonia C. S. & Junoy, Juan 2010 |
Lineus acutifrons
| Gibson R 1994: 88 |
| Gibson R & Knight-Jones EW 1990: 153 |
| Gibson R 1982: 84 |
| Southern R 1913: 9 |