Kapsulotaenia chisholmae, Hugh I. Jones & Alain de Chambrier, 2016
|
publication ID |
https://doi.org/10.5281/zenodo.155139 |
|
DOI |
https://doi.org/10.5281/zenodo.5579890 |
|
persistent identifier |
https://treatment.plazi.org/id/03FB87B7-FFF6-182B-FEC0-30A8FE8A0B11 |
|
treatment provided by |
Plazi |
|
scientific name |
Kapsulotaenia chisholmae |
| status |
sp. nov. |
Kapsulotaenia chisholmae n. sp.
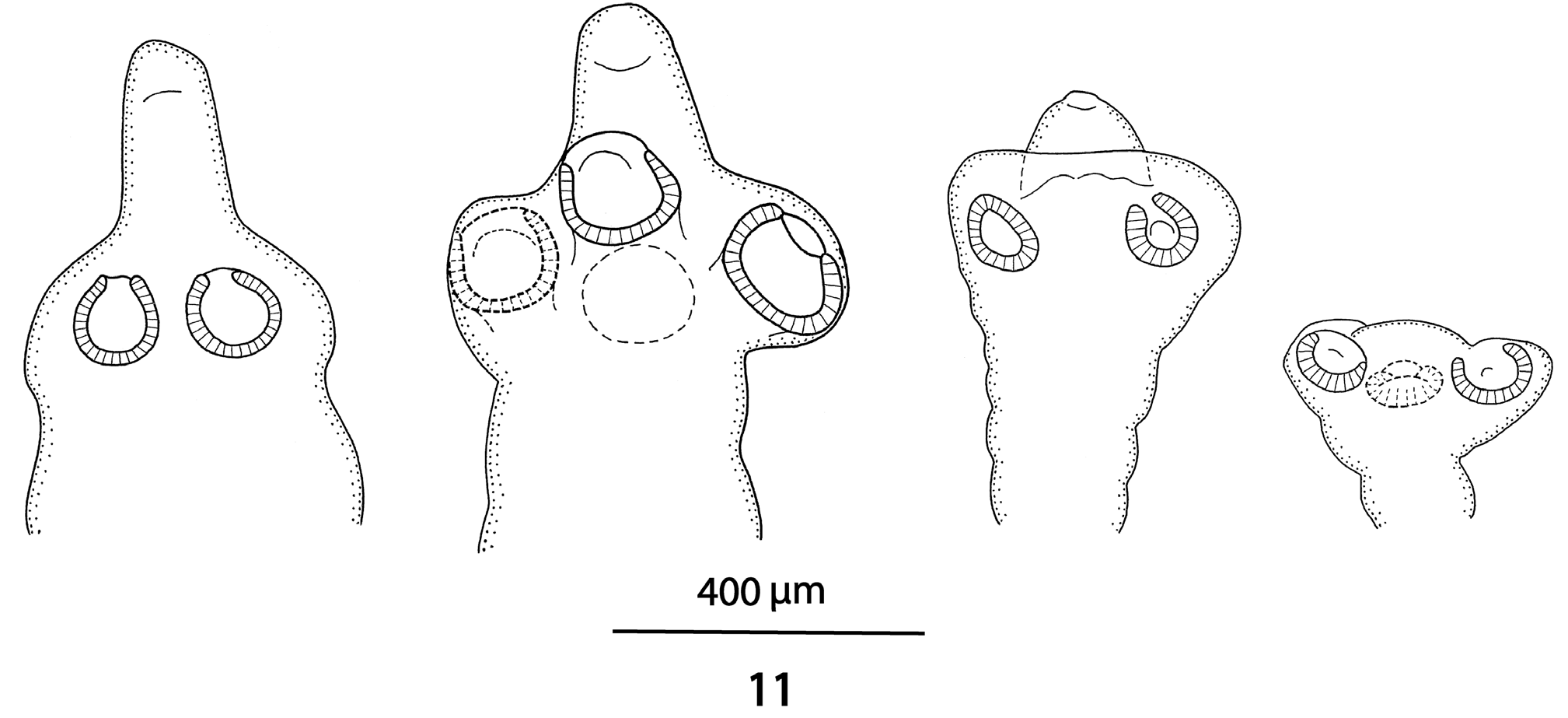
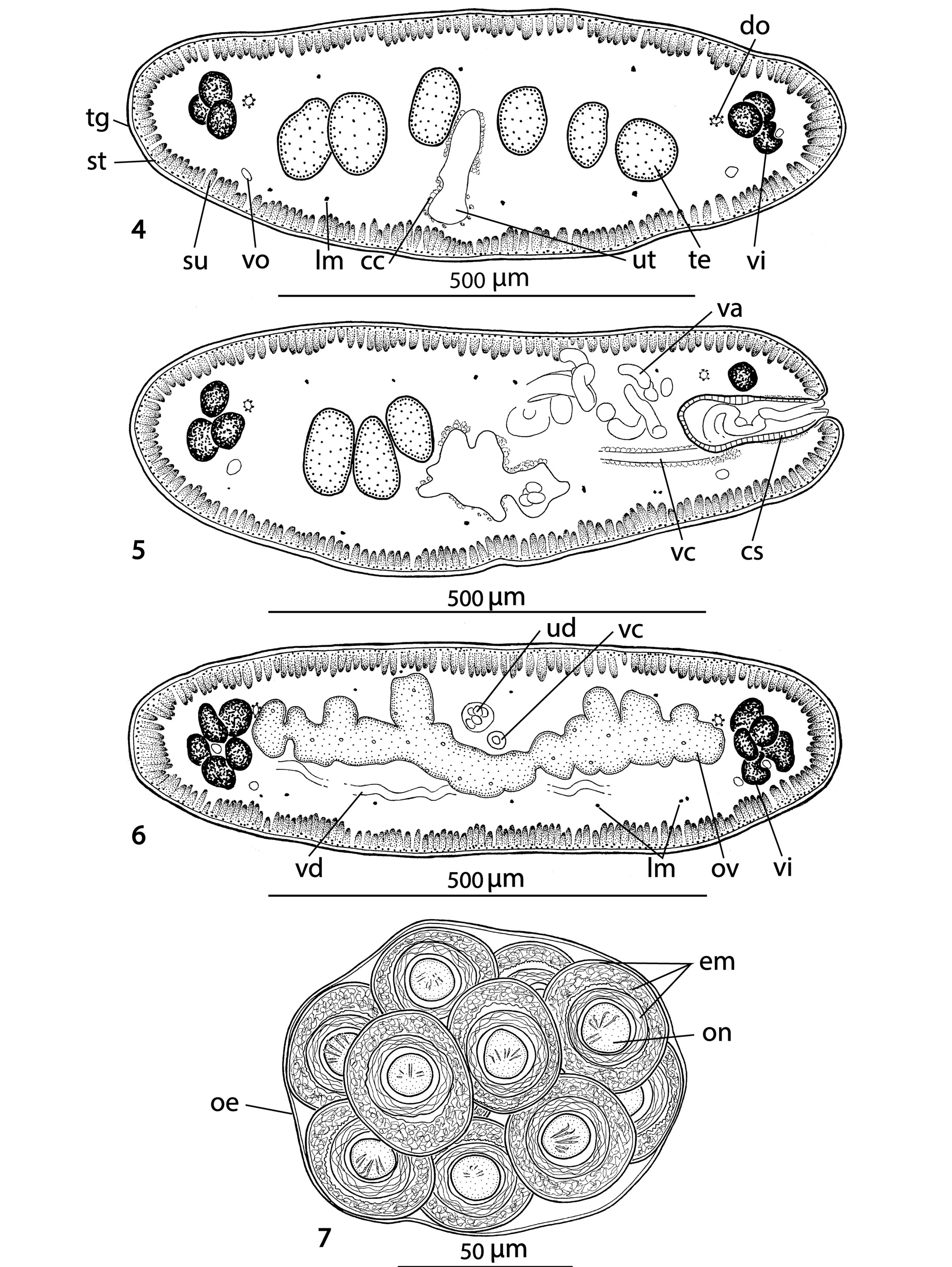
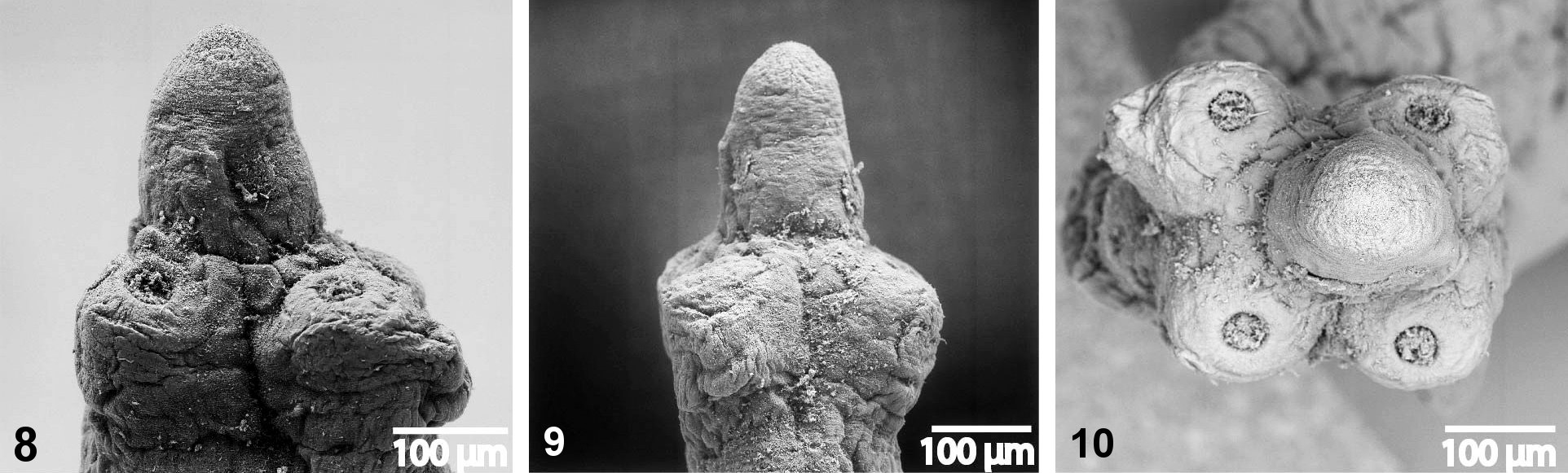
Figs 1-11 View Figs 1 - 3 View Figs 4 - 7 View Figs 8 - 10 View Fig 11
Material examined
Holotype: QM G235015 ; 5 slides of whole mounted specimen from host QM J47127 View Materials ; Central-west Queensland, Australia; no date recorded .
Paratypes: QM G235016 , 5 slides of whole mounted specimen from host QM J41654 View Materials ; Coorabulka Station , SW Queensland, 24°07’ S, 140°07’E. GoogleMaps ‒ QM G235017 , 3 slides of whole mounted specimen; 7 km West of Nelia , Queensland 20°39′0″S, 142°13′0″E, collected 01.11.2000. GoogleMaps ‒ QM G235018 , 3 slides of cross sections; 7 km West of Nelia , Queensland 20°39′0″S, 142°13′0″E, collected 01.11.2000. GoogleMaps ‒ MHNG-PLAT-31201 , 3 slides of whole mounted specimen and 12 slides of cross sections; 7 km West of Nelia , Queensland 20°39′0″S, 142°13′0″E, collected 01.11.2000 GoogleMaps .
Additional specimens: QM G235019 ; 3 specimens from host QM J15694 View Materials ; no date recorded .
Type locality: Central-west Queensland, Australia.
Type host: Varanus spenceri Lucas & Frost, 1903 . Reptilia, Varanidae . Queensland Museum accession no. J47127 View Materials .
Site of infection: Upper small intestine.
Prevalence of infection: Five out of 14 examined hosts were infected (36%).
Etymology: The species is dedicated to Leslie Chisholm, South Australian Museum, Australia.
Description (based on holotype and two paratypes; measurements in micrometres unless otherwise stated): Strobila acraspedote, anapolytic, total length 180- 315 mm, maximum width 840, body dorsoventrally flattened, early proglottides short and wide, immature proglottides wider than long to longer than wide, mature proglottides approximately twice as long as wide, 1.30 to 1.95 mm long, 715-840 wide, with pregravid and gravid proglottides longer than wide (1.8-2.3 mm long × 755-995 wide and 2.5-3.6 mm long × 875-1090 wide, respectively).
Scolex 340-450 wide, with pyramidal anterior retractile apex, almost twice as long as wide when totally everted, with apical organ 70-90 in diameter, and width at base about 130 ( Figs 1 View Figs 1 - 3 , 8-11 View Figs 8 - 10 View Fig 11 ). Four circular suckers, appear to face anteriorly, 90-160 in diameter. Rostellum-like retractable ( Fig. 11 View Fig 11 ). Small gladiate spinitriches on neck and proglottides. Proliferation zone about 140 μm wide. Very fine layer of subtegumentary longitudinal muscles, with well-stained subtegumentary cells. Internal longitudinal muscles formed by 8-10 bundles of muscle fibres; bundles absent on lateral sides of proglottides ( Figs 4-6 View Figs 4 - 7 ). Two pairs of osmoregulatory canals situated between testes and vitelline follicles, with dorsal canals slightly medial to ventral ones. Ventral canals thinwalled, 15-18 μm wide, sometimes overlapping vitelline follicles. Dorsal canals thick-walled (surrounded by thin muscle fibres), about 5-10 μm wide ( Figs 4-6 View Figs 4 - 7 ).
Testes medullary ( Figs 4, 5 View Figs 4 - 7 ), spherical to oval, often slightly elongated (40 × 24), in one or two layers, in two almost separated lateral fields ( Figs 2, 3 View Figs 1 - 3 ) total number 89-132, (x=108, n=10); 36-52 preporal testes, 5-12 postporal testes and 44-76 aporal testes. Vas deferens strongly coiled, directed anteriorly, reaching to midline of proglottis, often crossing it ( Figs 2, 3 View Figs 1 - 3 ). Cirrus-sac thick-walled, 175-200 long and 70-85 wide, representing 20-27% (x=24%, n=12) of proglottis width. Cirrus occupies about 40% of length of cirrus-sac. Genital atrium narrow, deep; genital pores irregularly alternating, post-equatorial, situated at 66-77% (x=71%, n=12) of proglottis length ( Figs 2, 3 View Figs 1 - 3 ).
Ovary medullary, bilobed, with dorsal lobes penetrating to cortex ( Fig. 6 View Figs 4 - 7 ), with narrow isthmus and follicles on dorsal side; ovary 385-630 wide, occupying 57-65% (x=62%, n=12) of proglottis width ( Figs 2, 3 View Figs 1 - 3 ). Relative ovarian size (ratio of ovarian size in relation to that of entire proglottis – see de Chambrier et al., 2012 for methods of measuring) 6.9-7.5% of proglottis size. Vagina anterior (93%) or posterior (7%, n=55) to cirrussac, lined with stained cells in its terminal (distal) part, without observable vaginal sphincter. Mehlis’ gland 50- 95 in diameter, representing 7-10% of proglottis width. Vitelline follicles oval, small, arranged in two lateral bands on each side of proglottis ( Figs 2, 3 View Figs 1 - 3 ), interrupted on poral side ventrally at level of terminal genitalia (cirrus-sac and vagina). Vitelline follicles do not reach anterior or posterior margin of proglottides, occupying 92-98% of proglottis length on poral side and 92-97% on aporal side ( Figs 2-3 View Figs 1 - 3 ).
Primordium of uterine stem medullary, already present in immature proglottides as an elongated structure formed by a thick layer of chromophilic cells. Formation of uterus of type 1 according to de Chambrier et al. (2004): uterine stem tipped with conspicuous concentration of numerous intensely stained cells ( Fig. 2 View Figs 1 - 3 ); lumen appears in first mature proglottides. Formation of lateral diverticula begins before appearance of eggs in uterus. Thin-walled lateral diverticula grow in pregravid and gravid proglottides, occupying up to 75% of proglottis width. Gravid proglottides with 300- 350 egg clusters. In gravid proglottides, ovaries, Mehlis’ gland, testes and vas deferens diminished in size, whereas cirrus-sac still visible. Lateral uterine branches difficult to observe.
8- 13 eggs in spherical to oval clusters, 100-125 x 95 -110, surrounded by smooth, thin membrane (outer envelope) closely apposed to eggs ( Fig. 7 View Figs 4 - 7 ). Eggs spherical, with a three-layered embryophore, with thick external layer, 37- 45 in diameter, intermediate layer nucleate of irregular shape 35-40 in diameter, internal layer envelope well developed, 23-28 in diameter. Oncosphere spherical, 12- 15 in diameter, with three pairs of embryonic hooks, 6-7 long ( Fig. 7 View Figs 4 - 7 ).
REMARKS
Due to the fact that the majority of cestodes were recovered from preserved lizards, most specimens of K. chisholmae were not in optimal condition: longitudinal excretory canals were sometimes difficult to distinguish, the uterine lateral diverticula in gravid proglottides were unclear, and the irregular shape and size of the testes may have been an artifact of poor preservation. The specimen collected from a living host in Nelia is better preserved, but does not have the scolex. Nonetheless, several constant features could be identified which readily differentiate this species from the other species of Kapsulotaenia ( Table 1 View Table 1 ).
de Chambrier (2006) recognized five species which have been formally described, viz. Kapsulotaenia tidswelli ( Johnston, 1909) from Varanus varius in eastern Australia ( Johnston, 1909), K. sandgroundi ( Carter, 1943) from the Komodo dragon, V. komodoensis ( Carter, 1943) , K. frezei Schmidt & Kuntz, 1974 from V. salvator (= V. palawanensis Koch, Gaulke & Böhme, 2010 ) in Palawan, Philippines (see Koch et al., 2010; Schmidt & Kuntz, 1974), K. saccifera ( Ratz, 1900) , from Varanus sp. in New Guinea ( Ratz, 1900a, b), and K. varia ( Beddard, 1913) from V. varius in Australia ( Beddard, 1913). Yamaguti (1959) however considered K. varia to be a synonym of K. sandgroundi , an opinion that the present authors do not share. Johnston (1909) commented that the scolex in K. saccifera is very similar to that in K. tidswelli .
The cestodes recovered from five out of fourteen V. spenceri in the present study resemble K. sandgroundi , as described by Carter (1943) and redescribed by de Chambrier (2006). However, Kapsulotaenia chisholmae n. sp. differs from this species and from K. varia and K. tidswelli by the anterior position of the vagina to cirrus-sac. It also differs from K. varia , K. frezei and K. saccifera by a different egg number in each cluster ( 8-13 in K. chisholmae versus 12-20, 90-100 and more than 100, respectively), from K. frezei and K. saccifera by a different cluster shape (spherical to oval versus banana-shaped in the two latter), from K. sandgroundi and K. tidswelli by a greater diameter of the embryophore (37-45 μm versus respectively 25-30 μm and 19-32 μm); from K. sandgroundi and K. varia ( sensu Nybelin, 1917 – see below), by the absence of a vaginal sphincter. Finally, K. chisholmae differs from K. varia , K. tidswelli , K. frezei and K. saccifera by a larger length of the strobila (up to 315 mm versus 30 mm, 27-30 mm, 40 mm, and 10-40 mm, respectively) ( Table 1 View Table 1 ).
DISCUSSION
Within the cestode order Proteocephalidea, Freze (1963) erected the subfamilyAcanthotaeniinae, with three genera Acanthotaenia Linstow, 1903 , Rostellotaenia Freze, 1963 and Kapsulotaenia Freze, 1963 , all of which occur in monitors ( Varanus spp.) ( Freze, 1965). de Chambrier & de Chambrier (2010) described another acanthotaenine genus, Vandiermenia, a parasite of the red-bellied snake Pseudechis porphyriacus from Australia. Kapsulotaenia is characterized principally by the presence of eggs in clusters in the gravid proglottides. Based on molecular data, de Chambrier et al. (2004) separated four species in this genus in Australia, with one species in V. varius ( White, 1790) , two in V. gouldii ( Gray, 1838) and one in V. rosenbergi Mertens, 1957 . There is also one species in V. indicus ( Daudin, 1802) (A. de Chambrier, unpublished). Species of Kapsulotaenia are believed to exhibit oioxenous specificity for their host ( sensu Euzet & Combes, 1980). The main problem in the study of the Kapsulotaenia species is that most descriptions are incomplete. Ratz (1900a, b) gave an identical, very poor description of Ichthyotaenia saccifera (= K. saccifera ) in two different publications, one in French and the other in German. Schwarz (1908) redescribed this taxon, but both descriptions are incomplete (missing a description of the scolex, number of testes, cirrus-sac ratio, testis field, uterine branches number, etc.) and no illustration was presented. Furthermore, the host was not identified to species. But Schwarz (1908) described for K. saccifera egg clusters elongated, bag-shaped (banana-shaped), each proglottis containing 2 to 11 clusters, which allows us to differentiate this taxa from our new species. Pending a revision of the genus, we consider K. saccifera as a species inquirenda.
Beddard (1913) described Acanthotaenia varia (= Kapsulotaenia varia ) from Varanus varius in a “Zoological Garden”. Nybelin (1917) collected some cestode specimens from Varanus gouldii that he identified as Acanthotaenia varia Beddard, 1913 . However, the description of these worms differs from the ones incompletely described by Beddard (1913) in the position of the vagina relative to the cirrussac (posterior in Beddard’s description and anterior/ posterior in Nybelin’s) and in the absence of a vaginal sphincter in the former specimens but present in the latter ( Table 1 View Table 1 ). Therefore, the specimens identified as Kapsulotaenia varia ( Beddard, 1913) described by Nybelin (1917) are considered as Kapsulotaenia sp. Furthermore, de Chambrier et al. (2004, 2015) observed a strict (oioxenous) specificity for the members of the monophyletic Kapsulotaenia and described differences between Kapsulotaenia sp. 2 from Varanus gouldii and Kapsulotaenia sp. 4 from V. varius . Johnston (1909) described K. tidswelli (as Acanthotaenia tidswelli ) from Varanus varius , but the same author ( Johnston, 1912) listed this species also as parasite of Varanus gouldii . However, this host record should be confirmed (voucher specimens are not available). It is obvious that the genus Kapsulotaenia is pending taxonomic revision.
The ecology and distribution of Varanus komodoensis (host of K. sandgroundi ) and V. spenceri (host of K. chisholmae ) differ. V. komodoensis only occurs in the Lesser Sunda islands, Indonesia, principally Komodo and Flores, where it inhabits deciduous monsoon rain forest, savannah and mangrove ( Ciofi, 2004). As well as feeding on invertebrates and smaller vertebrates, adult V. komodoensis can kill and consume large vertebrates such as deer, wild boar and goats; decomposing carcasses form a significant part of its diet ( Auffenberg, 1981). In contrast, V. spenceri is a burrowing species confined to relatively treeless grasslands with Astrebla spp. in central Queensland and the adjacent Northern Territory, and feeds on other smaller reptiles, small mammals such as Rattus villosisimus , and invertebrates such as orthopterans including plague locust species ( Pengilley, 1981; Valentic & Turner, 1997; Wooley, 2010). It is interesting to note that no cestodes were recovered from 12 V. gouldii and 10 V. panoptes in Queensland (H.I. Jones, unpublished).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Acanthotaeniinae |
|
Genus |