Fungomyza Roháček, 1999
|
publication ID |
https://doi.org/ 10.5281/zenodo.4272829 |
|
publication LSID |
urn:lsid:zoobank.org:pub:E95E58A5-E0F1-4237-9D7C-4A81BB3120DD |
|
DOI |
https://doi.org/10.5281/zenodo.4339801 |
|
persistent identifier |
https://treatment.plazi.org/id/03FB87A9-FFC6-FFA1-FE5A-6AE4FBA4FBA0 |
|
treatment provided by |
Felipe |
|
scientific name |
Fungomyza Roháček, 1999 |
| status |
|
Genus Fungomyza Roháček, 1999 View in CoL View at ENA
Fungomyza Roháček, 1999d: 391 View in CoL [feminine]; ROHÁĆEK & BARBER (2004): 132 –133 (redennition); ROHÁĆEK (2006a): 214 –215 (redescription); ROHÁĆEK (2009): 84–93, 110–111 (redescription, phylogeny); ROHÁĆEK & TÓTHOVÁ (2014): 169 –170 (phylogeny).
Type species. Opomyza albimana Meigen, 1830: 107 (original designation).
Diagnosis. (1) Head distinctly higher than long. (2) Eye large, broadly oval, with longest diameter almost vertical. (3) Frons moderately broad; frontal triangle variable in length, shining or sparsely microtomentose. (4) Frontal lunule small but always distinct. (5) Antenna geniculate between pedicel and 1st nagellomere, the latter strongly compressed laterally. (6) Arista short-ciliate. (7) Palpus yellow, slender but somewhat clavate, with 1 longer subapical seta. Cephalic chaetotaxy: (8) pvt relatively long, crossed or strongly convergent; (9) vte, vti and oc long; (10) 3 ors, anterior slightly or distinctly shorter; 1 microsetula in front of anterior ors; (11) postocular setulae short, in single row; (12) 1 relatively short vi, subvibrissa indistinguishable from peristomal setulae; (13) peristomal setulae small and variable in number. (14) Thorax slightly narrower than head, more or less shining, despite some microtomentum. Thoracic chaetotaxy: (15) 1 hu, 2 npl (anterior longer); (16) 1 medium-sized sa, 1 longer pa; (17) 1 distinct, relatively long prs; (18) 2 postsutural dc, both long and strong; (19) ac microsetae small and numerous, in 4 rows, ending in front of level of posterior dc; (20) 2 sc (apical strong, laterobasal short); (21) 1 minute upcurved ppl; (22) 2 distinct stpl (anterior shorter) and a few setulae in dorsal half of sternopleuron. (23) At least one pair of femora variegated (yellow and brown); (24) f 1 with relatively short ctenidial spine; (25) t 2 with distinct ventroapical seta; (26) male f 3 with posteroventral row of setae, distal of which are short and thick. (27) Wing long, moderately narrow; (28) wing membrane unicolourous. (29) C with inconspicuous spinulae among nne setulae on Cs 2; (30) R 2+3 long, parallel to C, ending nearer than or at the same distance from apex of R 4+5 as M; (31) R 4+5 slightly bent to sinuate; (32) cell dm relatively long; cross-vein r-m situated distal to middle of dm cell. (33) CuA 1 ending near, A 1 far from wing margin. (34) Anal lobe and alula well developed, relatively large.
Male abdomen. (35) T1 separate from T2, at least dorsally; (36) T2–T5 large and broad. (37) S1–S5 of variable width, dark but slightly paler than associated terga. Male postabdomen: (38) T6 short, transverse, bare and well sclerotized although paler-pigmented than S6–S8. (39) S6 and S7 strongly asymmetrical, partly fused and situated laterally, each with a few setulae. (40) S8 unusually long, less asymmetrical, more setose and situated dorsally.
Male genitalia. (41) Epandrium large, relatively densely setose, with 2–3 pairs longer. (42) Anal nssure very small, narrowly rounded triangular. (43) Medandrium high, slightly to conspicuously narrowed dorsally. (44) Cercus simple in shape, variable in size. (45) Gonostylus with 2–3 denticles on apex, and with external micropubescence partly or entirely reduced. (46) Hypandrium with anterior nat lobes not or slightly projecting dorsally; (47) transandrium with a forked or medially divided caudal process. (48) Pregonite large, nat, fused to hypandrium, with 2 groups of setae. (49) Postgonite simple, slender, long and relatively straight. (50) Phallapodeme with normal apex and shortly bifurcate symmetrical base. (51) Phallophore short, with more or less distinct ventral process; (52) distiphallus composed of distally membranous saccus and slender sclerotized nlum. (53) Saccus with relatively small membranous part, distinct basal and internal sclerites, and unarmed or with adpressed spinulae and/or setulae on surface; (54) nlum formed by 2 long, dark, band-like sclerites fused basally and apically, with simple apex. (55) Aedeagal part of folding apparatus with reduced connecting sclerite and its external wall provided with small grains, tubercles or short spines combined with dark striae. (56) Basal membrane below caudal process with dense small teeth or spines. (57) Ejacapodeme small to medium-sized, with subterminal digitiform process or modined (in some species).
(58) Female abdomen relatively shining, with broader terga (T2–T6) and narrower sterna (S2–S5). (59) Postabdomen long, basally broad, caudally strongly tapered, telescopically retractable from 7th segment, with less sclerotized and paler T8, S8, T10 and S10. (60) T6 broad, S6 relatively small, narrow. (61) T7 and S7 separate, S7 small, narrow, paler than T7. (62) T8 simple, relatively nat, pale-pigmented; (63) S8 narrow, medially longitudinally divided; its posterior bare parts dorsally curved and far invaginated into 8th segment. (64) Internal sclerites of female genital chamber (uterus) weakly sclerotized and reduced, formed by 1–2 pairs of partly fused posterior plates and (65) 1 anteroventral, broad but thin, incomplete and unpigmented annular sclerite. (66) Anterior part of uterus provided with an elongate, vesicular, weakly sclerotized ventral receptacle terminating in short projecting apex of various ending. (67) Accessory glands relatively small, on slightly dilated ringed ducts. (68) Spermathecae (1+1) roughly cup-like to pyriform, characterized by deep terminal invagination and narrowed basal part covered by nne spinulae; spermathecal ducts short. (69) T10 long and narrow, bare except for 1 pair of dorsal setae; (70) S10, simple, pale, slightly longer than T10, almost without micropubescence. (71) Cercus long and very slender, with a number of longer nne setae.
Discussion. The above generic diagnosis follows ROHÁĆEK (2009a), including the most ancestral E. Palaearctic relative, F. cercata Roháček, 2009 . Although outer appearance and several other features indicate the superncial similarity of Fungomyza to Paranthomyza Czerny, 1902 , Carexomyza or Quametopia (e.g. 1, 14, 53, 54, 58), previous morphological ( ROHÁĆEK 2009a) and molecular (ROHÁĆEK et al. 2009) phylogenetic analyses connrmed its association with the Anthomyza clade (see Fig. 137 View Figs 136–138 in ROHÁĆEK 2009a). Based on these phylogenetic hypotheses, there is support for a sister-group relationship between Fungomyza and a lineage consisting of Arganthomyza and Ischnomyia as here redenned. The relationship of Fungomyza to this sister pair seems to be supported by the following synapomorphies: caudal process of transandrium forked or medially desclerotized; female S8 longitudinally divided but with posterior bare parts recurved and deeply invaginated into 8th abdominal segment (strongest synapomorphy); spermathecal ducts markedly shortened (only in Arganthomyza , not in Ischnomyia ); well-sclerotized abdominal sterna; similar construction of ventral receptacle (see ROHÁĆEK 2009a). The sister clade of Arganthomyza + Ischnomyia is supported by modincation of the nlum of the distiphallus (sclerites expanded and attached or partly fused) and partial to complete coalescence of the female T7 and S7 to form a compact syntergosternite. The poorly known genus Receptrixa could also be a candidate for a closer (but highly modined) relative of all the above-discussed genera (shared features: short spermathecal ducts, distally positioned cross-vein r-m), but because the male of its only species is unknown this hypothesis cannot be properly tested.
The most recent hypothesis of phylogenetic relationships of anthomyzid genera based on multigene analyses ( ROHÁĆEK & TÓTHOVÁ 2014) surprisingly separated Fungomyza (represented by its type species, the European F. albimana ( Meigen, 1830)) as a sister group to all remaining analysed taxa of the family, thus excluding it from the Anthomyza clade. This relatively well-supported nnding contradicts the earlier molecular study of ROHÁĆEK et al. (2009) and, particularly, the current morphological knowledge (see above). Hitherto, we do not know of any morphological synapomorphy supporting the monophyly of the clade with all these (remaining) taxa. They, however, differ from Fungomyza in having microsaprophagous larvae, feeding on at least partly rotten tissues of plants, while those of Fungomyza species are mycophagous, developing in sporocarps of macrofungi (see DELY- DRASKOVITS 1972, CHANDLER 1978, ROHÁĆEK 2009a). The other possibility is that Fungomyza actually diverged basally from the common stem of the Anthomyzinae as the results of ROHÁĆEK & TÓTHOVÁ (2014) indicate; in this case, however, the former hypotheses based on morphological data would have to be incorrect (probably due to misinterpreted polarity of some characters).
The most diagnostic characters of Fungomyza (those apomorphic are marked “A” in parentheses) are: (1) eye with longest diameter almost vertical; (12) subvibrissa not developed (A); (17) 1 long prs; (23) at least one pair of femora variegated (bicolourous) (A); (32) cross-vein r-m situated distal to middle of cell dm; (38) male T6 short but well sclerotized; (40) male S8 unusually long (A); (42) anal nssure very small, narrowly rounded triangular (A); (43) medandrium high, more or less narrowed dorsally; (51) phallophore short, with more or less distinct ventral process; (53) saccus of distiphallus with membranous part having adpressed surface spinulae (A); (63) S8 narrow, longitudinally divided and invaginated into 8th segment (A); (64) internal paired sclerites of female genital chamber (uterus) weakly developed; (65) internal looped structure (homologous to annular sclerite) nne, non-sclerotized (?A); (68) spermatheca with terminal invagination (A). Most of the apomorphic features listed above are not unique for this genus – some of them are shared with related genera of the Anthomyza clade, especially Arganthomyza . The larval mycophagy of Fungomyza (though unconnrmed in F. cercata ) can be considered a non-morphological autapotypic feature of this genus ( ROHÁĆEK 2006a).
Fungomyza species can appear externally similar to other darkly coloured and relatively shining Anthomyzidae , including some Arganthomyza and Anthomyza species, Receptrixa receptrix ( Roháček & Freidberg, 1993) , Paranthomyza nitida and Carexomyza and Quametopia species. Fungomyza can be differentiated from these taxa by having more or less variegated legs, a distinctively reduced subvibrissa, sparse ac microsetae and a distally positioned r-m (the latter only known in Receptrixa ). The recently described Reliquantha variipes most closely resembles Fungomyza species, especially F. buccata (including similar variegation of legs) but R. variipes lacks a ctenidial spine on f 1 and has a distinct subvibrissa, in addition to many other structural differences in the male and female terminalia (see ROHÁĆEK 2013c and discussion under F. buccata below).
Three species included: Fungomyza cercata Roháček, 2009 from the Far East of Russia is considered a sister species of the remaining two, viz. F. albimana ( Meigen, 1830) (W. Palaearctic) and F. buccata Roháček & Barber, 2004 (E. Nearctic) . The latter two species are distributed on opposite sides of the Atlantic Ocean. This is a clear example of a sister-pair transatlantic distribution, where the more ancestral species is Nearctic ( ROHÁĆEK 2009a).
Key to identincation of Fungomyza species (world)
1 Fore femur and tibia uniformly yellow. Gonostylus small, externally without micropubescence, and apex with 3 denticles ( ROHÁĆEK 2009a: Fig. 125 View Figs 125–127 ); male cercus large and with thick dark setae ( ROHÁĆEK 2009a: Fig. 123 View Figs 117–124 ). Aedeagal part of folding apparatus with dense dark-pigmented spines and striae ( ROHÁĆEK 2009a: Fig. 127 View Figs 125–127 ). Female unknown. ..................................................... F. cercata Roháček, 2009 View in CoL ( Russia: Far East)
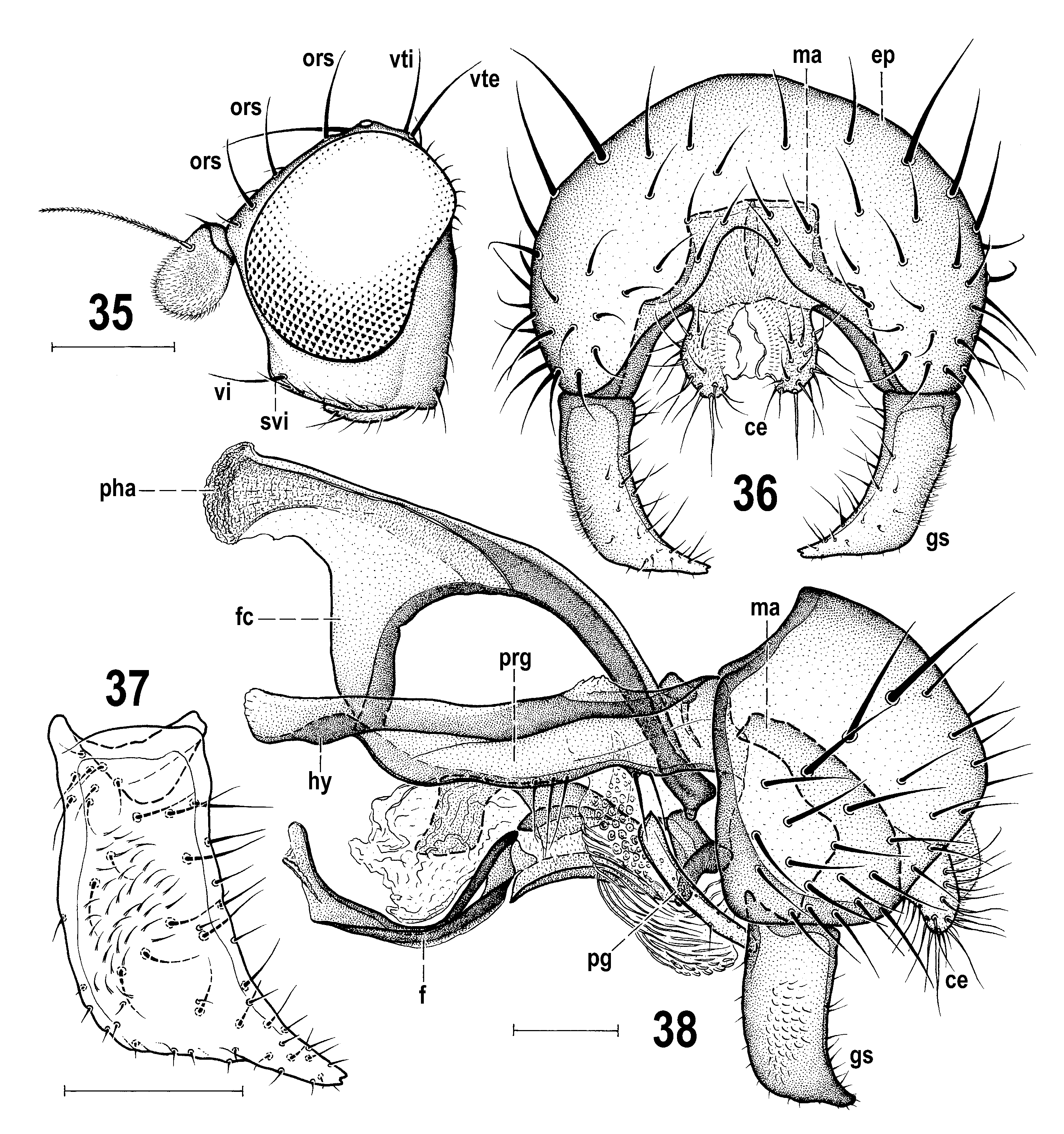
– Fore femur and tibia yellow and dark brown variegated ( ROHÁĆEK 2006a: Fig. 466 View Figs 460–466 ; OHÁĆEK 2009a: Fig. 147 View Figs 146–149 ). Gonostylus larger, externally at least partly micropubescent, and tapered apex with 2 denticles ( Fig. 37 View Figs 35–38 ; ROHÁĆEK 2006a: Fig. 468 View Figs 467–473 ); male cercus small, nnely and pale setose ( Fig. 36 View Figs 35–38 ; ROHÁĆEK 2006a: Fig. 467 View Figs 467–473 ). Armature of aedeagal part of folding apparatus pale-pigmented, inconspicuous. .......................................... 2
2(1) Anterior ors long and gena unusually high ( Fig. 35 View Figs 35–38 ); frons, including most of frontal triangle, microtomentose and dull. Mid and hind femora darkened on distal half. Gonostylus with posteromedially projecting apex ( Fig. 37 View Figs 35–38 ) and pregonite without posterior projection ( Fig. 38 View Figs 35–38 ). Female T8 short and transverse ( Fig. 42 View Figs 42–46 ); spermathecae more rounded ( Fig. 46 View Figs 42–46 ). ........................ F. buccata Roháček & Barber, 2004 View in CoL (eastern USA)
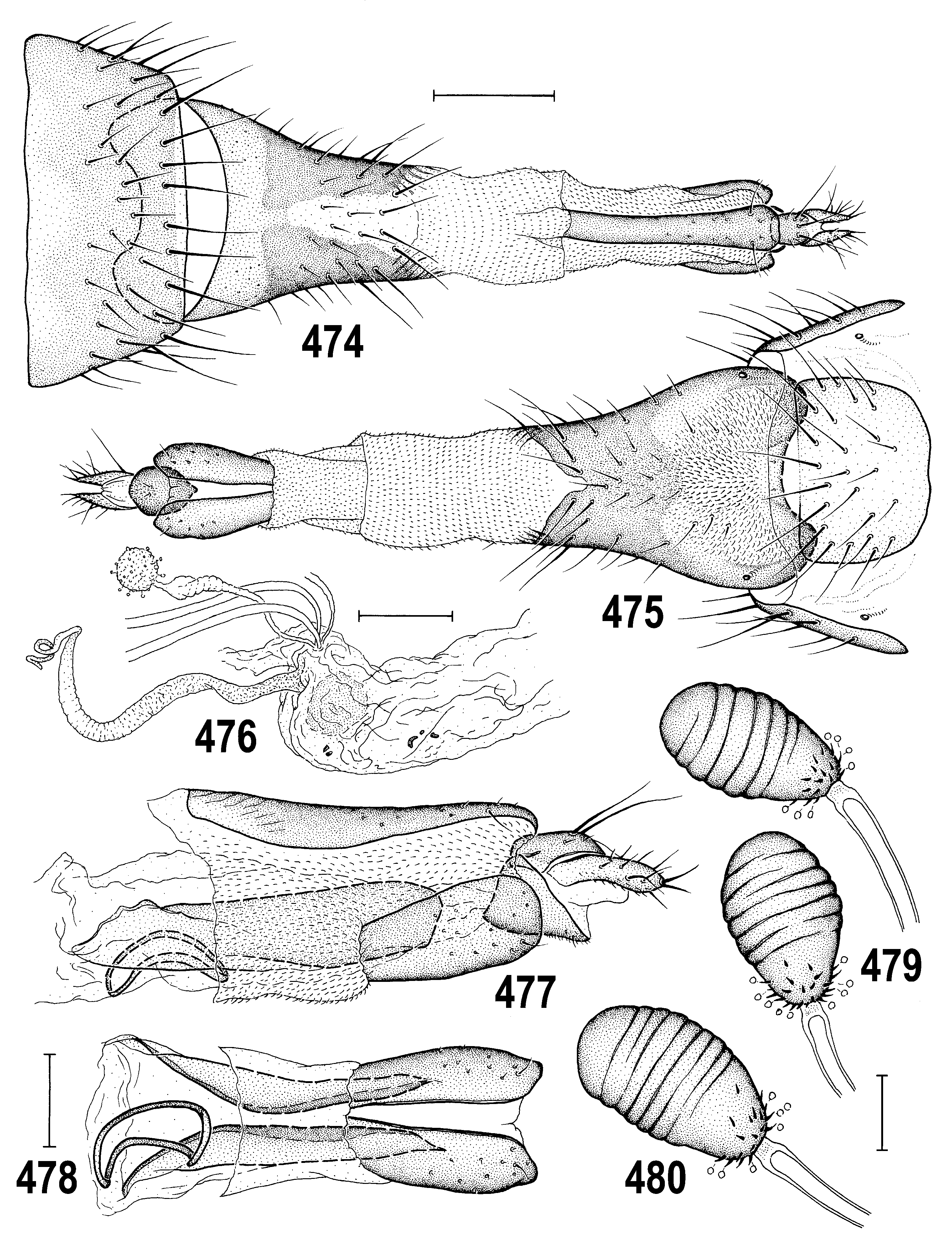
– Anterior ors short and gena very low ( ROHÁĆEK 2009a: Fig. 147 View Figs 146–149 ); frontal triangle and posterior part of orbit bare and shining. Mid and hind femora uniformly yellow (OHÁĆEK 2009a: Fig. 147 View Figs 146–149 ). Gonostylus apically narrowed but not projecting and pregonite with distinct posterior projection ( ROHÁĆEK 2006a: Figs 468, 471 View Figs 467–473 ). Female T8 longer than broad; spermathecae roughly pyriform with narrowed basal part ( ROHÁĆEK 2006a: Figs 474, 476 View Figs 474–480 ). ..................................... F. albimana ( Meigen, 1830) (Europe, Turkey)
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Fungomyza Roháček, 1999
| Roháćek, Jindřich & Barber, Kevin N. 2016 |
Fungomyza Roháček, 1999d: 391
| ROHACEK J. & TOTHOVA A. 2014: 169 |
| ROHACEK J. 2006: 214 |
| ROHACEK J. & BARBER K. N. 2004: 132 |
| ROHACEK J. 1999: 391 |