Geoplana multicolor Graff, 1899
|
publication ID |
https://doi.org/10.5281/zenodo.207332 |
|
DOI |
https://doi.org/10.5281/zenodo.5628130 |
|
persistent identifier |
https://treatment.plazi.org/id/03FB5633-FFC3-FFE5-94FA-FB1B8E74FD9E |
|
treatment provided by |
Plazi |
|
scientific name |
Geoplana multicolor Graff, 1899 |
| status |
|
Geoplana multicolor Graff, 1899
Geoplana multicolor Graff, 1899 (Tafel VII, Figs. 12–14 View FIGURES 10 – 13 View FIGURES 14 – 17 : external aspect). Geoplana multicolor: Marcus, 1951
Geoplana multicolor: Froehlich, 1955
Geoplana multicolor: Froehlich, 1956a
Geoplana multicolor: Froehlich, 1956b
Geoplana multicolor: Froehlich, 1957
Geoplana sp. 7: Leal-Zanchet & Carbayo, 2000 Geoplana sp. 3: Castro & Leal-Zanchet, 2005
Material examined. EMF Nr. 1–4: São Paulo, SP, Brazil – Four specimens collected and studied for the first time by E. Marcus. EMF 1: Pharynx and copulatory apparatus: sagittal sections on 4 slides/ Figs. 176 and 177 ( Marcus, 1951: p. 187); EMF 2: copulatory apparatus: sagittal sections on 4 slides; EMF 3: copulatory apparatus: sagittal sections on 2 slides; EMF 4: pharynx and copulatory apparatus: sagittal sections on 5 slides; MZU PL.00081: São Francisco de Paula, RS, Brazil – R. Murowaniecki, leg. 04.V.1998 – Pre-pharyngeal region: transverse sections on 4 slides; pharynx: sagittal sections on 12 slides; copulatory apparatus: sagittal sections on 19 slides; MZU PL.00082: São Francisco de Paula, RS, Brazil – A. Leal-Zanchet, coll. 04.V.1998 – preserved in ethanol 70%; MZU PL.00083: São Francisco de Paula, RS, Brazil – G. Fiorentin, leg. 30.V.1998 – Anterior region in three fragments: horizontal sections on 6 and 9 slides, respectively, and transverse sections on 19 slides; pre-pharyngeal region: transverse sections on 7 slides; pharynx: sagittal sections on 12 slides; copulatory apparatus: sagittal sections on 10 slides; MZU PL.00084: Santa Maria ( CISM), RS, Brazil – C. Cristofoli, leg. 19.XI.2000 – Pre-pharyngeal region: transverse sections on 6 slides; pharynx: sagittal sections on 17 slides; copulatory apparatus: sagittal sections on 16 slides; MZU PL.00085: Santa Maria ( CISM), RS, Brazil – A. Seitenfus, leg. 13.I.2001 – preserved in ethanol 70%; MZU PL.00086: Santa Maria (Três Barras), RS, Brazil – M. Fontoura, leg. 09.VI.2001 – preserved in ethanol 70%; MZU PL.00087: Ibarama, RS, Brazil – T. Santos, leg. 21.I.2001 – Pre-pharyngeal region: sagittal sections on 32 slides; pharynx: sagittal sections on 22 slides; copulatory apparatus: sagittal sections on 25 slides; MZU PL.00088: Santa Maria ( CISM), RS, Brazil – V. Baptista, leg. 29.IX.2001 – Anterior region in two fragments: sagittal sections on 25 slides; pre-pharyngeal region: transverse sections on 8 slides; pharynx: sagittal sections on 13 slides; copulatory apparatus: sagittal sections on 22 slides; MZU PL.00089: Santa Maria (Camobi), RS, Brazil – S. Cechin, leg. 29.IX.2001 – Pre-pharyngeal region: transverse sections on 5 slides; pharynx: sagittal sections on 9 slides; copulatory apparatus: sagittal sections on 9 slides; MZU PL.00090: Santa Maria ( CISM), RS, Brazil – V. Dias, leg. 24.I.2002 – Pre-pharyngeal region: transverse sections on 5 slides; pharynx: sagittal sections on 11 slides; copulatory apparatus: sagittal sections on 11 slides; MZU PL.00091: Santa Maria ( CISM), RS, Brazil – R. Castro, leg. 24.I.2002 – Anterior region at the level of the ovaries: horizontal sections on 36 slides; pre-pharyngeal region: transverse sections on 29 slides; pharynx: sagittal sections on 47 slides; copulatory apparatus: horizontal sections on 67 slides; MZU PL.00092: Santa Maria ( CISM), RS, Brazil – M. Fontoura, leg. 24.I.2002 – preserved in ethanol 70%; MZU PL.00093: Santa Maria (Pains), RS, Brazil – T. Santos, leg. 18.X.2001 – Pre-pharyngeal region: transverse sections on 5 slides; pharynx: sagittal sections on 17 slides; posterior region: sagittal sections on 11 slides; MZU PL.00094: Santa Maria (Três Barras), RS, Brazil – R. Castro, leg. 11.VII.2002 – preserved in ethanol 70%; MZU PL.00095: Caxias do Sul, RS, Brazil – R. Fleck, leg. 17.IX.2002 – Pre-pharyngeal region: transverse sections on 15 slides; pharynx: sagittal sections on 23 slides; copulatory apparatus: sagittal sections on 19 slides; MZU PL.00096: Santa Maria (Três Barras), RS, Brazil – R. Castro, leg. 15.XII.2002 – preserved in ethanol 70%; MZU PL.00097: Santa Maria (Três Barras), RS, Brazil – L. Matos, leg. 15.XII. 2002 – preserved in ethanol 70%; MZU PL.00098: Santa Maria (Três Barras), RS, Brazil – L. Matos, leg. 15.XII.2002 – Pre-pharyngeal region: transverse sections on 1 slide; pharynx: sagittal sections on 4 slides; copulatory apparatus: sagittal sections on 3 slides; MZU PL.00099: Santa Maria (Três Barras), RS, Brazil – V. Baptista, leg. 15.XII.2002 – preserved in ethanol 70%; MZU PL.00100: Santa Maria (Três Barras), RS, Brazil – R. Castro, leg. 23.VIII.2002 – Copulatory apparatus: sagittal sections on 30 slides.
Type locality. São Paulo state, Brazil.
Distribution. Rio de Janeiro (Teresópolis), São Paulo (Alto da Serra, Avaré, Mogi das Cruzes, São Paulo), Paraná (Curitiba, Guapiara, Ponta Grossa, Lapa), Rio Grande do Sul (São Francisco de Paula, Caxias do Sul, Ibarama, Santa Maria) – Brazil
Diagnosis. Dorsum brown with light median stripe, which may contain a median concentration of rust red or brownish pigment inside it, bordered by black paramedian stripes; eyes dorsal, with clear halos; conspicuous glandular margin; mc:h, 3–9%; pharynx cylindrical; esophagus: pharynx ratio, 13%–30%; foremost testes approximately level with ovaries, most posterior ones near root of pharynx; efferent ducts open laterally into anterior end of prostatic vesicle; prostatic vesicle consisting of two portions, a forked, extrabulbar, proximal portion with ample lumen and an unpaired, much narrower, distal portion which enters the bulbar muscular coat; male atrium ovalelongate, with almost the entire cavity occupied by the penis papilla; ejaculatory duct opens ventrally dislocated, near the tip of the truncate, asymmetrical papilla; oviducts emerging, dorsally and laterally dislocated, from median third of ovaries, and ascending behind gonopore; common glandular oviduct short; vagina as a dorso-anteriorly curved ental portion of female atrium, sometimes indiscernible; female atrium oval-elongate, obliquely disposed, with a narrowed lumen, presenting two distinct regions, the ental one lined by an epithelium with multilayered aspect; male atrium at least as long as female atrium; asymmetric gonopore canal; ample dorsal fold separating male and female atria which open to gonopore canal in different parasagittal planes.
Description. External morphology.
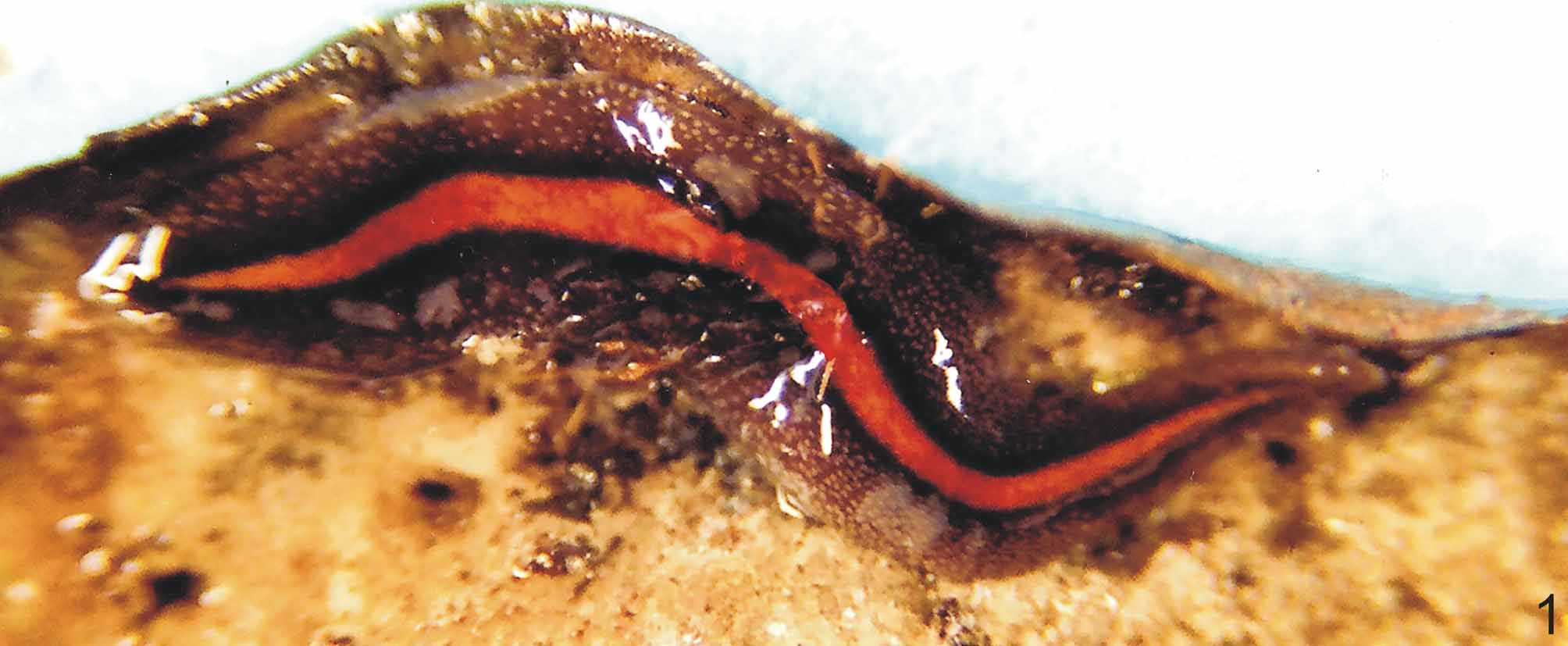
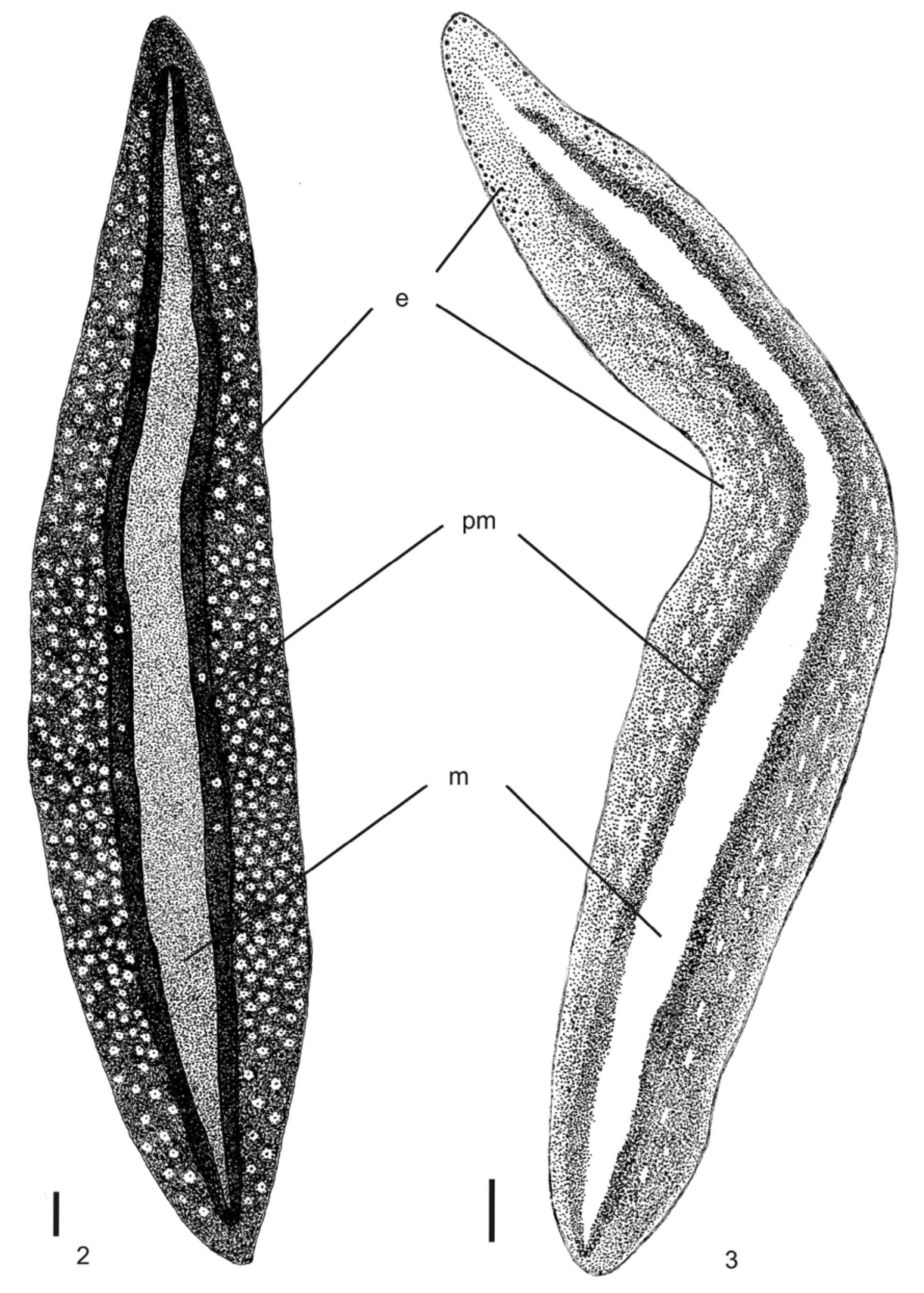
Body broad and flat, anterior end as well as posterior pointed. When crawling, maximal length reaches 55mm (Table 1). Mouth distance from anterior tip varies from 52% to 76%; gonopore between 62% and 83%, relative to body length (Table 1). Dorsal ground-colour brown; the venter is pale yellow or greyish with denser pigmentation at the margins. Dorsally, there is a broad, orange or reddish median stripe, whereby at the external limits a very dense pigment creates a black paramedian stripe on each side of the dorsum. Lateral to these, there is dark-brown pigmentation overlaying the dorsal ground colour ( Figs. 1–3 View FIGURE 1 View FIGURES 2 – 3 ). Specimens from São Paulo may contain a median concentration of rust red or brownish pigment in the middle of the broad median stripe.
In specimen MZU PL.00092, from Santa Maria ( Fig. 2 View FIGURES 2 – 3 ), median and paramedian stripes begin at approximately 1 mm from anterior tip (ca. 3% of body length) and extend up to 0.7 mm from posterior one (ca. 98% ofbody length). At median third of body, median and paramedian stripes, respectively, 1.2 mm and 0.5 mm wide (24% and 10% of body width, respectively). After fixation, the median stripe becomes pale yellow.
Eyes uniserially surround anterior tip, becoming pluriserial immediately after. In specimen MZU PL.00092, between 2 mm and 4 mm (approx. 12% and 45% of body length) behind anterior end, these form three series extending near to body margins. Between approx. 5 mm and 15 mm from anterior tip, dorsal eyes surrounded by clear halos become abundant. They occur up to paramedian stripes, sometimes invading these. Towards posterior end, they become less numerous ( Figs. 2–3 View FIGURES 2 – 3 ).
Internal morphology.
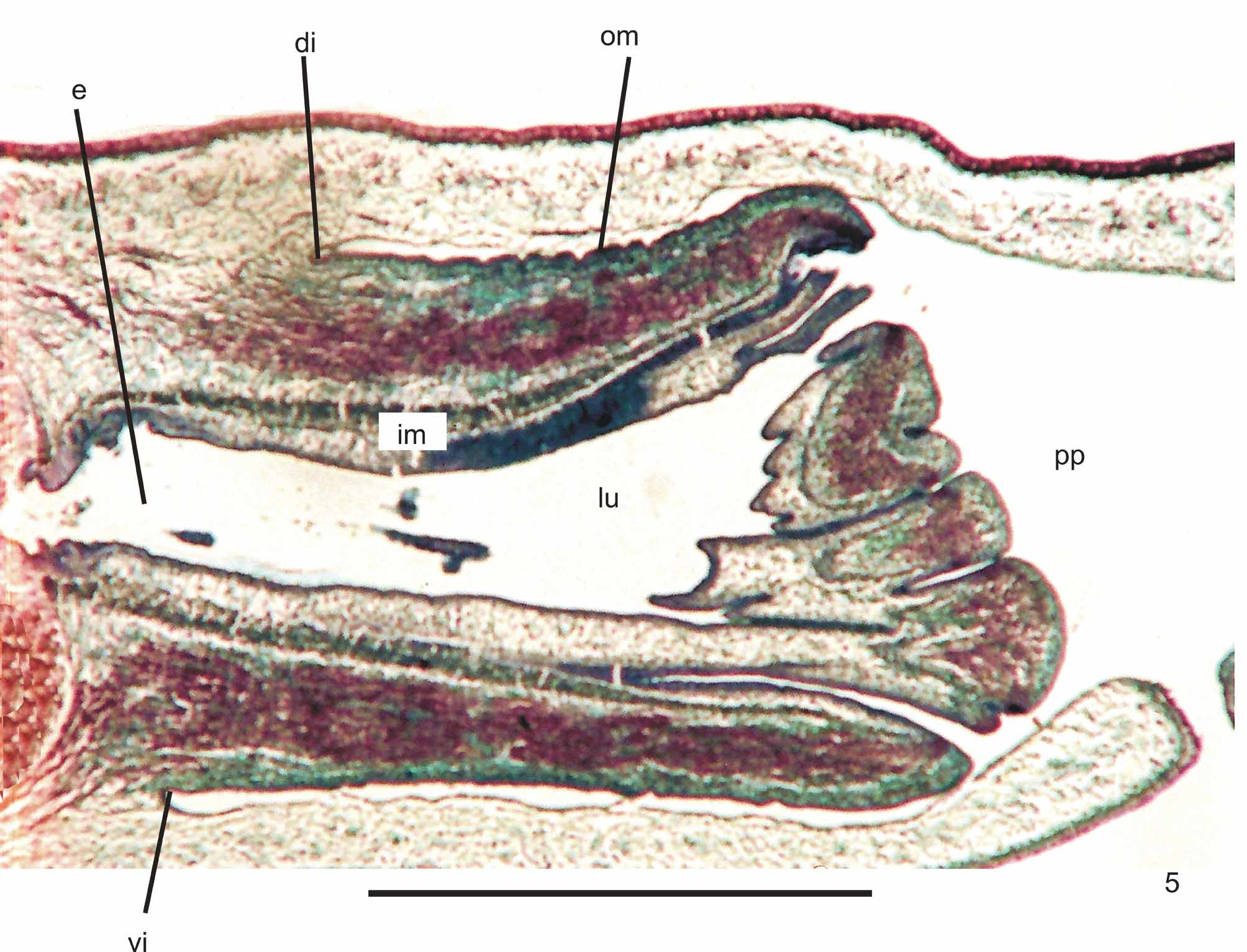
Epidermis and musculature at pre-pharyngeal region ( Fig. 4 View FIGURE 4 ): Creeping sole broad (Table 1). Three types of secretory cells open through dorsal epidermis and body margins: (1) rhabditogen cells with xanthophil secretion; (2) cells with coarse xanthophil secretion, more numerous towards body margins; (3) cells with xanthophil amorphous secretion and subepithelial bodies; and (4) cells less frequent with cyanophil amorphous secretion. Creeping sole receives abundant cells with cyanophil amorphous secretion and less numerous secretory cells of two types: rhabditogen cells and cells with coarse xanthophil secretion. Glandular margin constituted by numerous cells with coarse xanthophil secretion; cells with xanthophil amorphous secretion and subepithelial bodies; and some rhabditogen cells.
Cutaneous musculature with the usual three layers, longitudinal layer with small bundles, being approximately three times higher than the other two. At the sagittal plane, ventrally and dorsally with similar thickness. Towards body margins cutaneous musculature progressively lower. Mc:h 3% to 9% ( Table 2 View TABLE 2 ).
MZU MZU MZU MZU MZU MZU
PL.00081 PL.00083 PL.00088 PL.00091 PL.00093* PL.00095 Mesenchymatic musculature mainly composed of transversal, oblique, and dorsoventral fibres, the former ones may constitute a supra-intestinal transversal and a sub-intestinal transversal layer, each of them approx. 3–4 fibers thick. If existent, longitudinal fibers are indiscernible.
Pharynx ( Fig. 5 View FIGURE 5 ): Pharynx of cylindrical type with dorsal and ventral insertions almost at the same transversal level. Mouth near the end of pharyngeal pouch. Short esophagus lined with columnar ciliated epithelium presenting some insunk nuclei and coated with interwoven circular and longitudinal fibers. Pharynx and pharyngeal lumen lined with columnar ciliated epithelium showing some insunk nuclei, those of the pharyngeal lumen located among fibers of inner pharyngeal musculature. Pharyngeal glands with cell bodies located in mesenchyme, mainly anteriorly to pharyngeal pouch. Four secretory cell types: (1) cells with densely arranged, xanthophil granulous secretion; (2) cells with strongly erythrophil granulous secretion; (3) cells with cyanophil amorphous secretion; and (4) cells with strongly cyanophil, fine granulous secretion. Outer musculature of pharynx (ca. 19 µm thick) constituted of thin longitudinal subepithelial layer, followed by a thicker circular one, mixed internally with few longitudinal fibers. Towards pharyngeal tip, circular layer becomes as thin as longitudinal one. Inner pharyngeal musculature (ca. 20 µm thick) composed of thick circular subepithelial layer, mixed with some longitudinal fibers. Inner musculature gradually thins down towards pharyngeal tip. Esophagus:pharynx ratio from 13% to 34%.
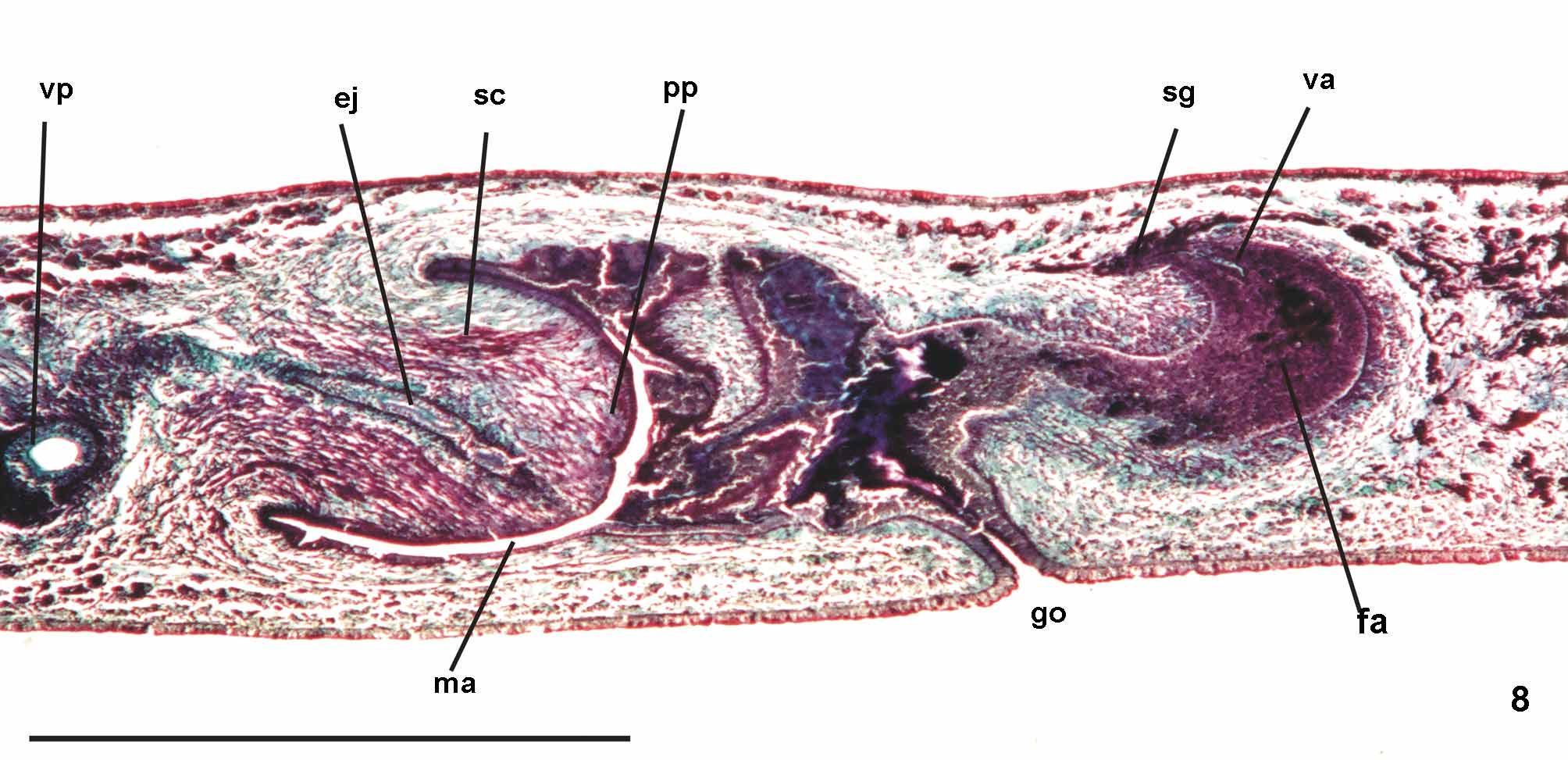
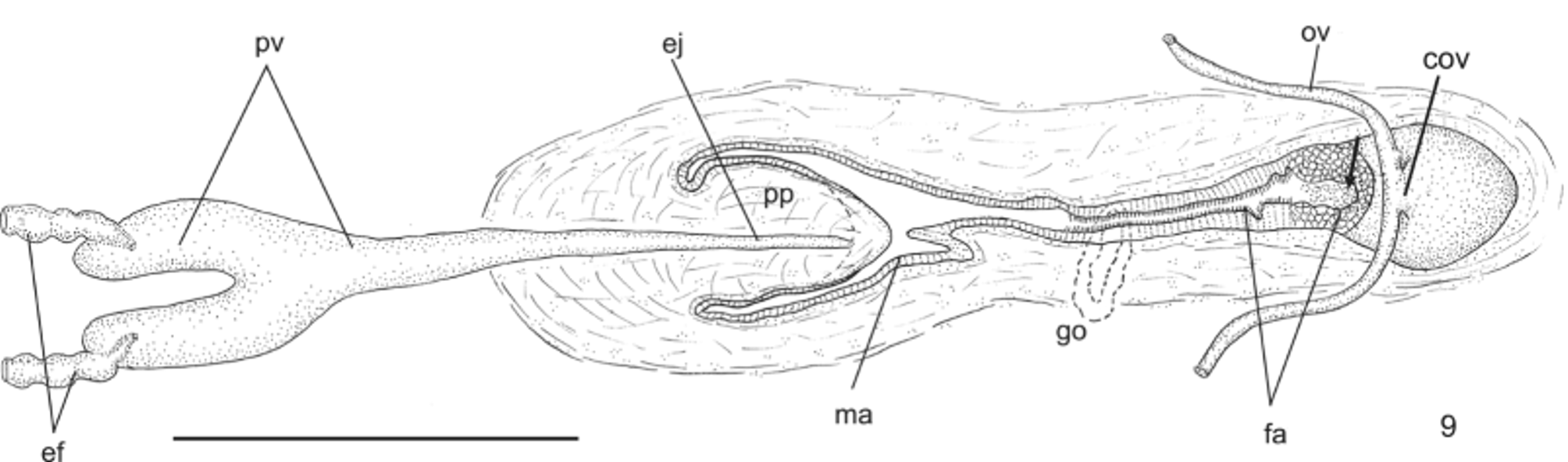
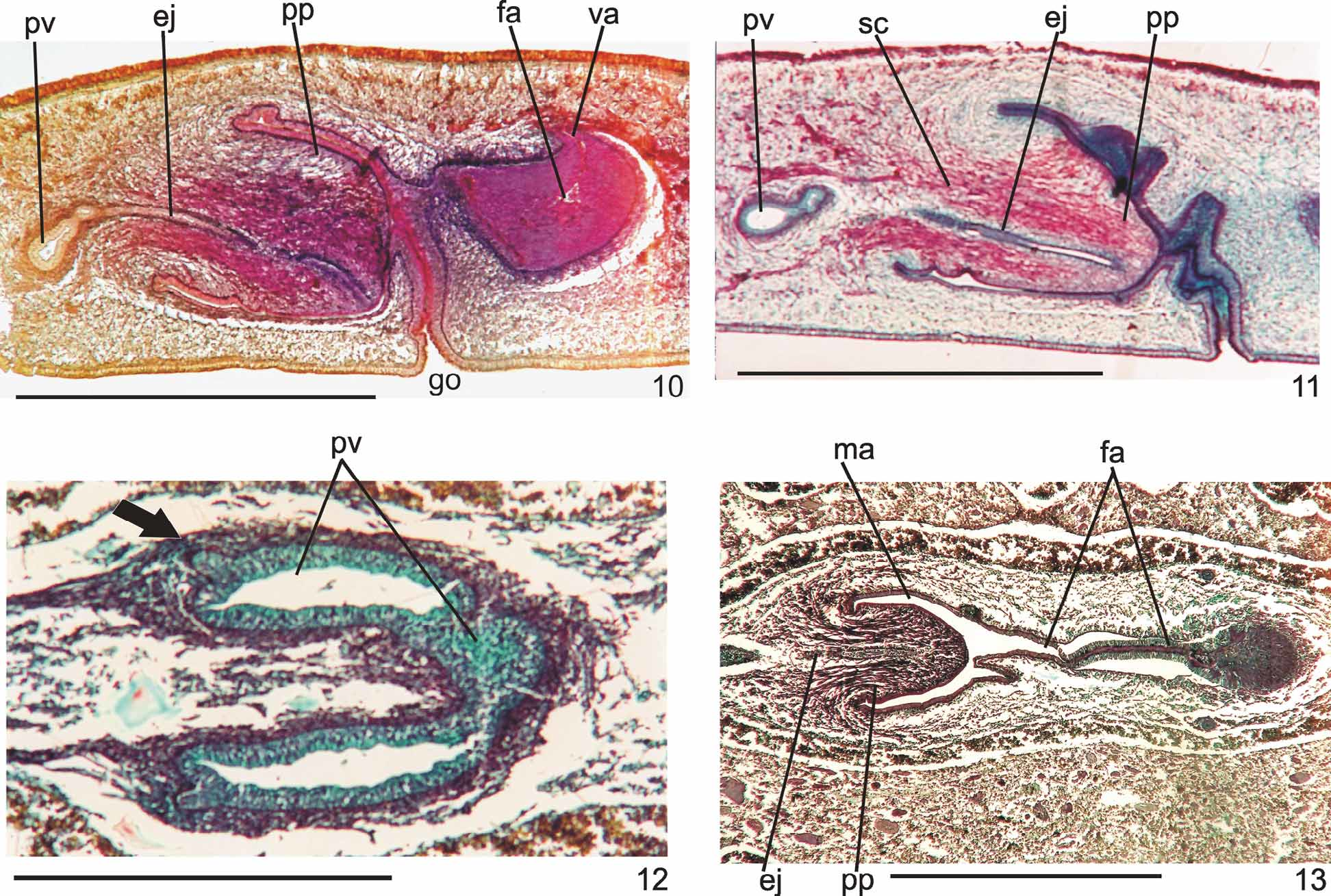
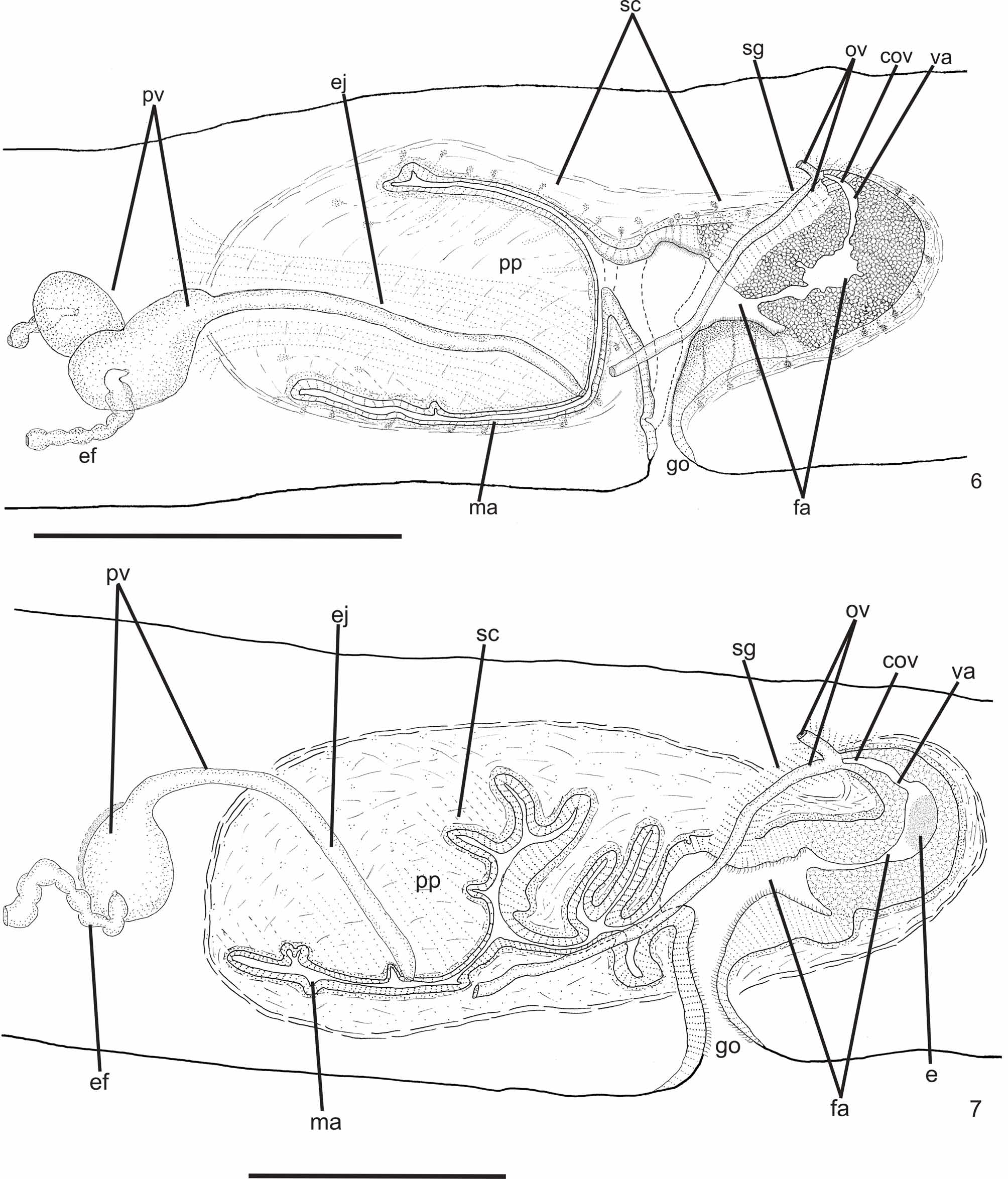
Reproductive apparatus: Testes begin at approximately the same level as ovaries and extend near to root of the pharynx (Table 1), constituting two irregular rows dorsal to the intestinal branches on each side of the body ( Fig. 4 View FIGURE 4 ). Pre-pharyngeally, efferent ducts dorsal to oviducts, sometimes laterally displaced. Behind and/or lateral to pharynx these form false seminal vesicles, opening laterally into anterior third of prostatic vesicle ( Figs. 6, 7 View FIGURES 6 – 7 , 9 View FIGURE 9 , 12 View FIGURES 10 – 13 ).
Prostatic vesicle comprises two portions, a forked, extrabulbar, proximal portion with ample lumen, and an unpaired much narrower distal portion which proceeds posteriorly and enters the bulbar muscular coat, continuing into the ejaculatory duct. The latter traverses the truncate asymmetrical penis papilla to open, ventrally displaced, near its tip. Oval-elongate male atrium with almost the entire cavity or at least the ental half occupied by the penis papilla. Male atrium may contain folds in its ectal portion (Tables 1, 3, Figs. 6–11, 13 View FIGURES 6 – 7 View FIGURE 8 View FIGURE 9 View FIGURES 10 – 13 ).
Lining epithelium of efferent ducts cuboidal ciliated; thin muscularis (ca. 2–4Μm thick) mainly of circular fibers. Prostatic vesicle lined with irregular columnar to pseudostratified non-ciliated epithelium, gradually diminishing its height towards ejaculatory duct. Erythrophil cells with granular secretion and cyanophil cells with amorphous secretion, both with bodies lying in mesenchyme, mainly around vesicle, show numerous openings into the proximal portion and scarce openings into the distal one. Muscularis of vesicle (35–60 µm and 10–25 µm thick, respectively, in the proximal and distal portions) constituted of interwoven circular, oblique, and longitudinal fibers.
Ejaculatory duct lined with columnar ciliated epithelium, receiving numerous openings from secretory cells with amorphous, cyanophil secretion and subepithelial bodies. It is coated with muscularis (ca. 10–15 µm thick) of circular fibers interposed with some longitudinal ones. Penis papilla lined with columnar, non-ciliated epithelium, changing from cuboidal to squamous towards its tip, presenting insunk nuclei, mainly next to the tip. Three types of secretory cells run longitudinally in the papilla, with numerous openings through its lining epithelium: (1) cells with fine densely arranged granular slightly erythrophil secretion opening mainly at the tip of the papilla; (2) cells with granular heavily stained erythrophil secretion opening mainly laterally next to the papilla root; and (3) cells with cyanophil amorphous secretion and openings more numerous laterally next to the papilla root. A fourth type of cell, with xanthophil amorphous secretion and openings mainly at the tip of the papilla, occurs scarcely. Erythrophil cells present cell bodies external to common muscle coat; cyanophil and xanthophil cells, intrabulbar or intrapapillar cells bodies. Muscularis (5–23µm) mainly composed of circular layer with some mixed longitudinal fibers; thinner towards the tip of the papilla. Longitudinal, radial and oblique muscle fibers cross the papilla.
Epithelial lining of male atrium, columnar non-ciliated, becoming ciliated in the near of gonopore. Epithelial cells with xanthophil or erythrophil apical secretion, higher distally. Two types of secretory cells, with cell bodies internal to common muscle coat, empty through the epithelium: abundant cells with cyanophil amorphous secretion and cells with fine granulous erythrophil secretion. Muscularis weakly developed (8µm) throughout male atrium, constituted of circular subepithelial fibers and longitudinal subjacent ones.
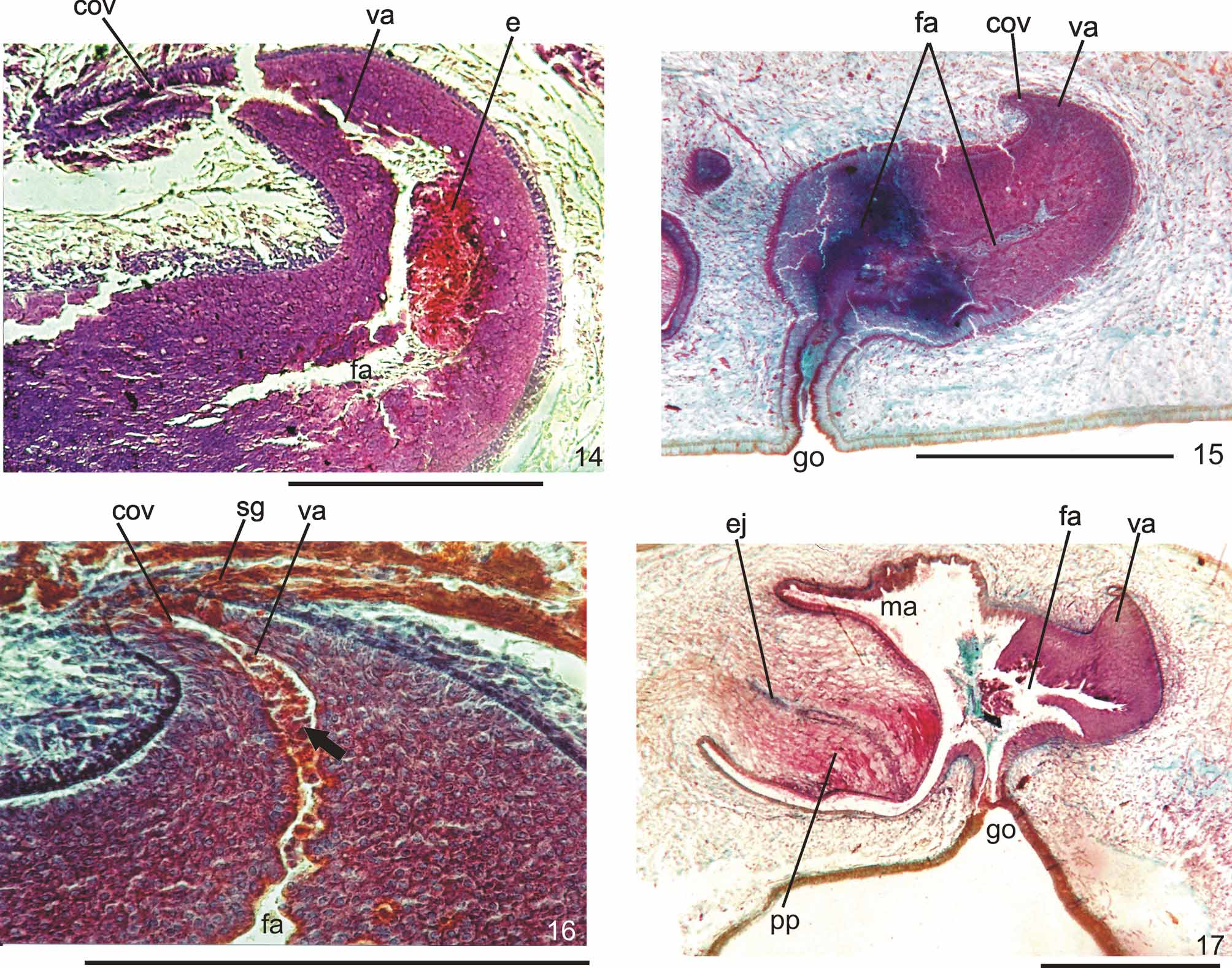
Ovaries pear-shaped. Oviducts emerge dorsally and laterally dislocated from median third of ovaries, then lead backwards immediately dorsal to nerve plate. Behind the gonopore, oviducts ascend posteriorly and medially inclined, to unite dorsally to the female atrium, thus forming the common glandular oviduct. A short common glandular oviduct opens into ental portion of female atrium. Female atrium obliquely disposed and oval-elongate in shape, and with an irregular lumen, narrower in the ental half. Length of female atrium equal to that of male atrium or approx. half male atrium length. Ental portion presents a short, dorso-anteriorly curved diverticulum (vagina) (Tables 1, 3, Figs. 6–10 View FIGURES 6 – 7 View FIGURE 8 View FIGURE 9 View FIGURES 10 – 13 , 14–17 View FIGURES 14 – 17 ).
Lining epithelium of paired oviducts cuboidal to columnar ciliated, of common oviduct columnar ciliated; muscle coat of paired and common oviducts mainly with circular fibers and some interposed longitudinal fibers. Shell glands with xanthophil or erythrophil secretion opening into distal ascending portion of paired oviducts, and also into common glandular oviduct.
Lining of vagina and ental portion of female atrium very thick with multilayered aspect; more irregular in height, changing to columnar ciliated, in the ectal portion of female atrium ( Figs. 6–8 View FIGURES 6 – 7 View FIGURE 8 , 14–16 View FIGURES 14 – 17 ). Epithelial cells of the proximal portion non-ciliated, presenting erythrophil cytoplasm; those of the distal portion with xanthophil or erythrophil apical secretion. Numerous secretory cells with cyanophil amorphous secretion and less frequent cells with granulous erythrophil or xanthophil secretion discharge into female atrium. Cell bodies of both glands are mainly internal to the common muscle coat, those of cyanophil cells may occur externally to this coat. Muscularis of female atrium constituted of interwoven circular and longitudinal fibers, thicker (20–40µm) than in male atrium. Muscularis of vagina mainly of circular fibers with some interposed longitudinal fibers (16–30µm).
Gonopore canal approximately vertical in sagittal plane, asymmetric, inclining forwards or backwards to communicate with male or female atria which open to the canal in different parasagittal planes. A dorsal fold approximately at the level of gonopore canal leads to venter and fuses with ventral wall ( Figs. 6–8 View FIGURES 6 – 7 View FIGURE 8 , 10, 11 View FIGURES 10 – 13 ). Gonopore canal lined with columnar ciliated epithelium with numerous openings of cells with cyanophil amorphous secretion, rhabditogen cells, and cells with granulous erythrophil secretion. Muscularis of circular fibers with some interposed longitudinal fibers.
Common muscle coat thin with circular, longitudinal and oblique fibers, inconspicuous around female atrium. Between the atrial muscularis and common muscle coat, a poorly developed stroma is present with muscle fibers variously oriented.
Remarks. Vitellaria were inconspicuous in specimens MZU PL.00090, MZU PL.00091, and MZU PL.00095. They were in an early stage of maturation in specimens MZU PL.00083 and MZU PL.00087, although well developed in specimens MZU PL.00081, MZU PL.00088 and MZU PL.00100. In specimens MZU PL.00081, MZU PL.00083, MZU PL.00088, MZU PL.00091 and MZU PL.00100 there was an abundant holocrine secretion produced by cells of a multilayered-like lining, in the lumen of the female atrium ( Figs. 8–10 View FIGURE 8 View FIGURE 9 View FIGURES 10 – 13 , 14, 16 View FIGURES 14 – 17 ). Specimens MZU PL.00088, MZU PL.00087 and MZU PL.00100 show signs of recent copulation. Specimen MZU PL.00088 had a great amount of erythrophil amorphous secretion, cyanophil amorphous secretion and sperm in the female cavity ( Fig. 8 View FIGURE 8 ), as well as sperm in the common glandular duct. Specimen MZU PL.00100 presented a more asymmetrical penis papilla, a highly folded male atrium and an ejaculate constituted of a very condensed mass of erythrophil secretion associated with sperm and cyanophil secretion fixed in the ental portion of the female atrium near the vagina ( Fig. 14 View FIGURES 14 – 17 ). The specimens MZU PL.00088 and MZU PL.00100, from Santa Maria, and MZU PL.00095, from Caxias do Sul, presented a longer male atrium than the specimens from other localities in Southern Brazil and those from São Paulo (Tables 1, 3). The very contracted and curled specimen MZU PL.00087 from Ibarama, directly fixed after falling into a pitfall, showed anatomical and histological characters similar to the specimens from other localities in Southern Brazil and those from São Paulo. It differed especially due to the ample communication between the male and female atria in the sagittal plane which seemed to be caused by its curled condition ( Fig. 17 View FIGURES 14 – 17 ). This specimen was in an early phase of maturation as the development of vitellaria and secretory cells denotes. Specimens MZU PL.00084, MZU PL.00089, MZU PL.00093 and MZU PL.00098 were juveniles. Specimens MZU PL.00093 and MZU PL.00098 showed only a cell-agglomerate encircling a tubular or ovoid organ lined by a columnar epithelium which is interpreted as the initial formation of the copulatory organs. In specimens MZU PL.00084 and MZU PL.00089, the copulatory organs were in an early stage, being lined by a columnar epithelium of variable thickness, becoming pluristratified in the female atrium of specimen MZU PL.00084. There were neither a penis papilla nor a separation between atria in both specimens, and the openings of the female and male ducts into the copulatory organs were indiscernible.
TABLE 2. Cutaneous musculature in the median region of a transverse section of the pre-pharyngeal region, and ratio of the height of cutaneous musculature to the height of the body (mc: h index) of specimens of Geoplana multicolor Graff, 1899. * Specimen collected in a pitfall trap and directly fixed in formaldehyde.
| Dorsal musculature 23 | 32 | 15 | 28 | 48 | 35 |
|---|---|---|---|---|---|
| Ventral musculature 27 | 37 | 12 | 22 | 34 | 43 |
| Mc:h 6% | 7% | 3% | 3% | 9% | 7% |
| CISM |
Verticillium dahliae from cotton |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Geoplaninae |
|
Genus |
Geoplana multicolor Graff, 1899
| Leal-Zanchet, Ana Maria & Matos, Lisiane Bernardes De 2011 |
Geoplana multicolor :
| Froehlich 1957 |
Geoplana multicolor :
| Froehlich 1956 |
Geoplana multicolor :
| Froehlich 1956 |
Geoplana multicolor :
| Froehlich 1955 |
Geoplana multicolor :
| Marcus 1951 |
Geoplana multicolor
| Graff 1899 |