Bathymodiolus septemdierum Hashimoto & Okutani, 1994
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5214.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:76F65EE8-5307-4B4C-AA5F-0B39FBCA5A48 |
|
DOI |
https://doi.org/10.5281/zenodo.7390465 |
|
persistent identifier |
https://treatment.plazi.org/id/03FA6E53-B848-5400-5994-90954F3CF82B |
|
treatment provided by |
Plazi |
|
scientific name |
Bathymodiolus septemdierum Hashimoto & Okutani, 1994 |
| status |
|
Bathymodiolus septemdierum Hashimoto & Okutani, 1994 View in CoL
Synonymy
1994 Bathymodiolus septemdierum Hashimoto & Okutani , [OD], Venus 53 (2), 72, (fig. 5, pl. 1.7-8, pl. 2.1, pl. 3.4), [type locality Mokuyo Seamount, Izu-Bonin Arc]; published August 31, 1994; holotype (NSMT Mo-70035) and paratypes as designated by Hashimoto & Okutani (1994), amended herein.
1994 Bathymodiolus brevior Cosel, Métivier & Hashimoto , The Veliger 37 (4), 375, (figs 1-10, 26, 30-34, 37, 38), [type locality Vailili vent site, Lau Basin]; published October 4, 1994.
2001 Bathymodiolus marisindicus Hashimoto, Venus 60, 142, (figs 2-9, 12), [type locality Kairei vent site, Central Indian Ridge].
Material Examined. Type material not examined for the three species for reasons presented in the Materials and Methods.
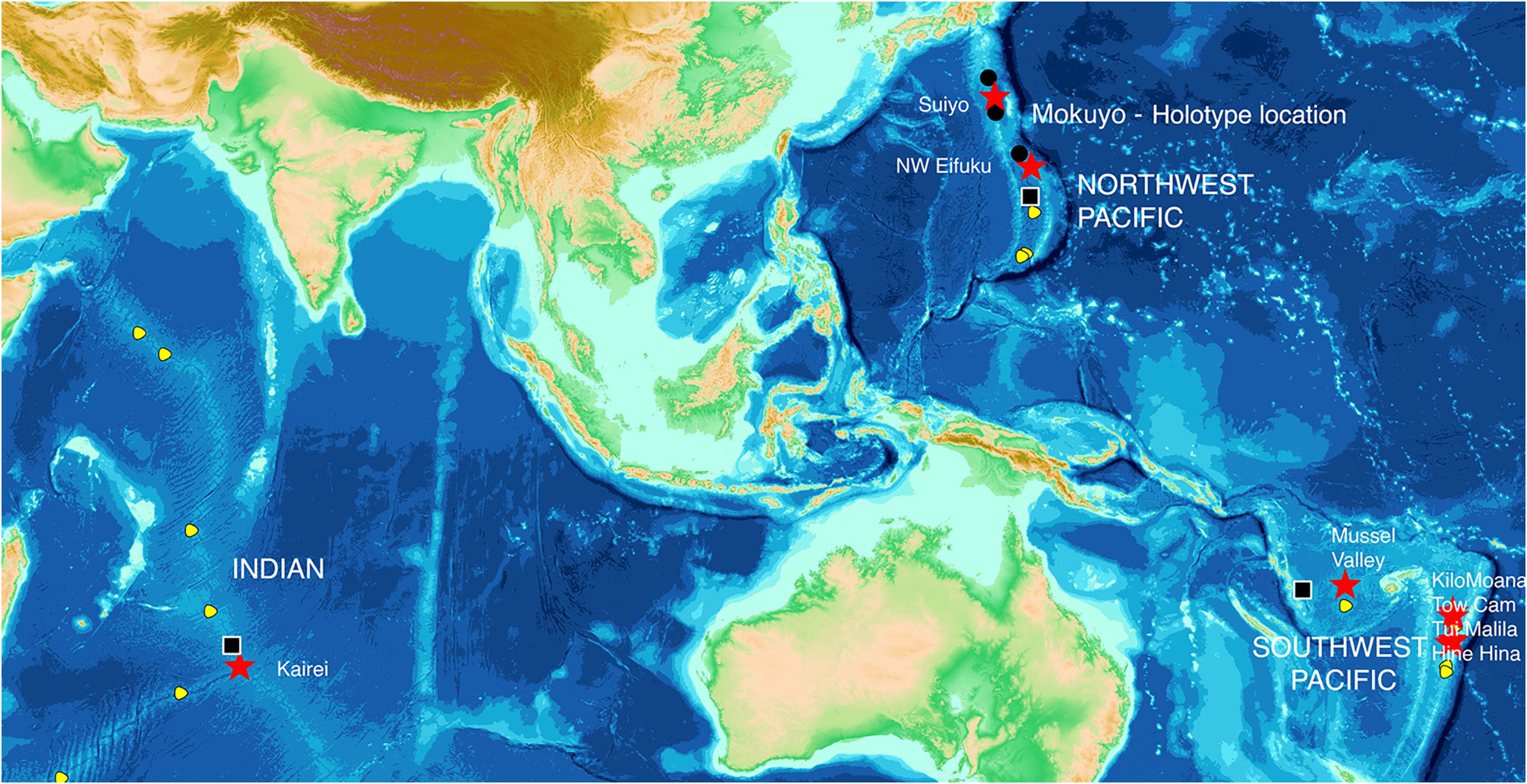
Northwest Pacific: Whole specimens of 41 mussels from the Izu-Bonin Arc: Mokuyo Seamount (type locality), Suiyo Seamount and Myojin Knoll (both paratype localities). Valves of 64 mussels from the Mariana Volcanic Arc: NW Eifuku and Nikko Seamounts. Whole specimens of 32 mussels from NW Eifuku Seamount.
Southwest Pacific: Valves of 67 mussels from four vent sites in the Lau Basin: Hine Hina (paratype locality for B. brevior ), Tow Cam, Tu’i Malila, and Kilo Moana. Eleven valves from one site in the North Fiji Basin: White Lady (material in B. brevior description).
Indian Ocean:Whole specimens of nine mussels from one site on the Central Indian Ridge: Kairei (type locality).
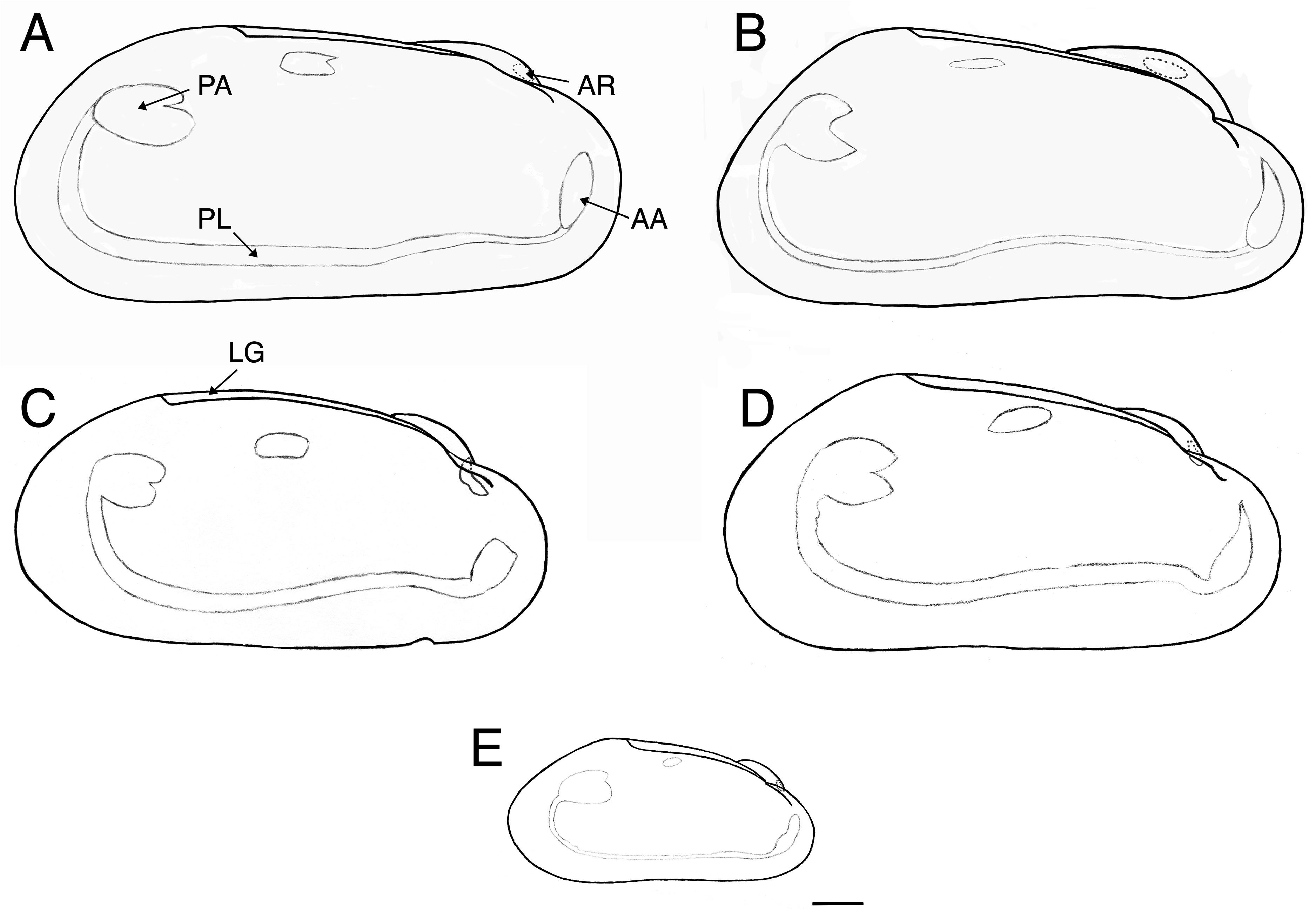
Description. Shell ( Fig. 2 View FIGURE 2 ). Modioliform shell of variable fragility: very thin to strongly calcified dependent on habitat. Size up to 151 mm in length; height to length ratio decreases with size varying from 0.40 to 0.55 in large specimens. Similarly, tumidity (height/width) varies from 1.0 to 1.3. Juveniles are more oval and slender. Ventral margin may be slightly convex, straight or slightly concave, with a population showing all three forms. Anterior margin broadly to narrowly rounded; posterior margin may be fully rounded or forming a sharp angle at the dorsal junction ( Fig. 3 View FIGURE 3 ). Beak subterminal positioned at about 15% of shell length but ranging from 8% to 22% in larger specimens.
Exterior smooth with largely intact glossy golden brown periostracum that may accumulate mineral deposits from vent fluids. Inner aragonite layer may show variable growth lines or signs of reabsorption/deposition, while outer calcite layer has distinct daily growth bands. Larval shell about 400 μm ( Cosel et al. 1994, figs 30-33). Hinge edentulous. Ligament opisthodetic extending 90 to100% of the posterodorsal margin in large specimens.
Body. Anterior adductor muscle scar usually situated anterior to umbo but may overlap; shape variable around an oval ( Fig. 3 View FIGURE 3 ); anterior retractor muscle scar usually under middle or forward end of the umbonal cavity. Anterior bundle scar of the posterior pedal/byssal retractor muscle located under ligament at half to two-thirds of its length. Posterior bundle of this muscle forms a bilobed scar with the posterior adductor muscle ( Fig. 3 View FIGURE 3 ). These muscle insertions can vary somewhat in position partly dependent on shell size.
Ctenidia large from two-thirds to five-sixths length of shell, variable within a population. Inner and outer demibranchs more or less equal length with the former attached to mantle anteriorly but detached posteriorly. Valvular siphonal membrane narrow, terminating at postero-ventral margin; papilla absent.
Mantle lobes separate along ventral margin usually with fluted edging. Mantle folds extend dorsally over anterior adductor muscle, along anterior margin, then down and posteriorly under the anterior adductor muscle. Foot somewhat variable in breadth with ventral byssal groove about two-thirds the length.
Posterior byssus retractor muscles arise from foot-byssus muscle complex to attach as two muscle bundles (anterior and posterior) on dorsal and postero-dorsal shell interior. Posterior pedal retractors stout rising from base of foot to join with anterior bundle of the posterior byssus retractor muscles. Anterior retractor muscles of the complex rising forward to attach on anterior wall of umbo.
Habitat and Distribution. Known only from hydrothermal vents of the western Pacific and Indian oceans ( Fig. 1 View FIGURE 1 ). Hosts thiotrophic bacterial symbionts in gills, thus requires access to sulphide-rich vent fluids. Usually forms dense clusters around diffuse fluid flows, attached by byssal threads to rocks or other shells. Where currents disperse concentrated fluids over the seabed, can form extensive areas of high biomass. Shells very thin where fluid pH low.
This mussel species has a wide range: Northwest Pacific in Izu-Bonin Volcanic Arc, Mariana Volcanic Arc and Mariana Back-arc spreading ridge; in the Southwest Pacific, in the New Hebrides Arc, North Fiji Basin, Lau Basin, Tonga-Tofua Arc, and Kermadec Arc; in the Indian Ocean on the Southwest Indian Ridge and the Central Indian Ridge. Thus, B. septemdierum occurs in mid-ocean ridge, back-arc ridge and volcanic arc settings.
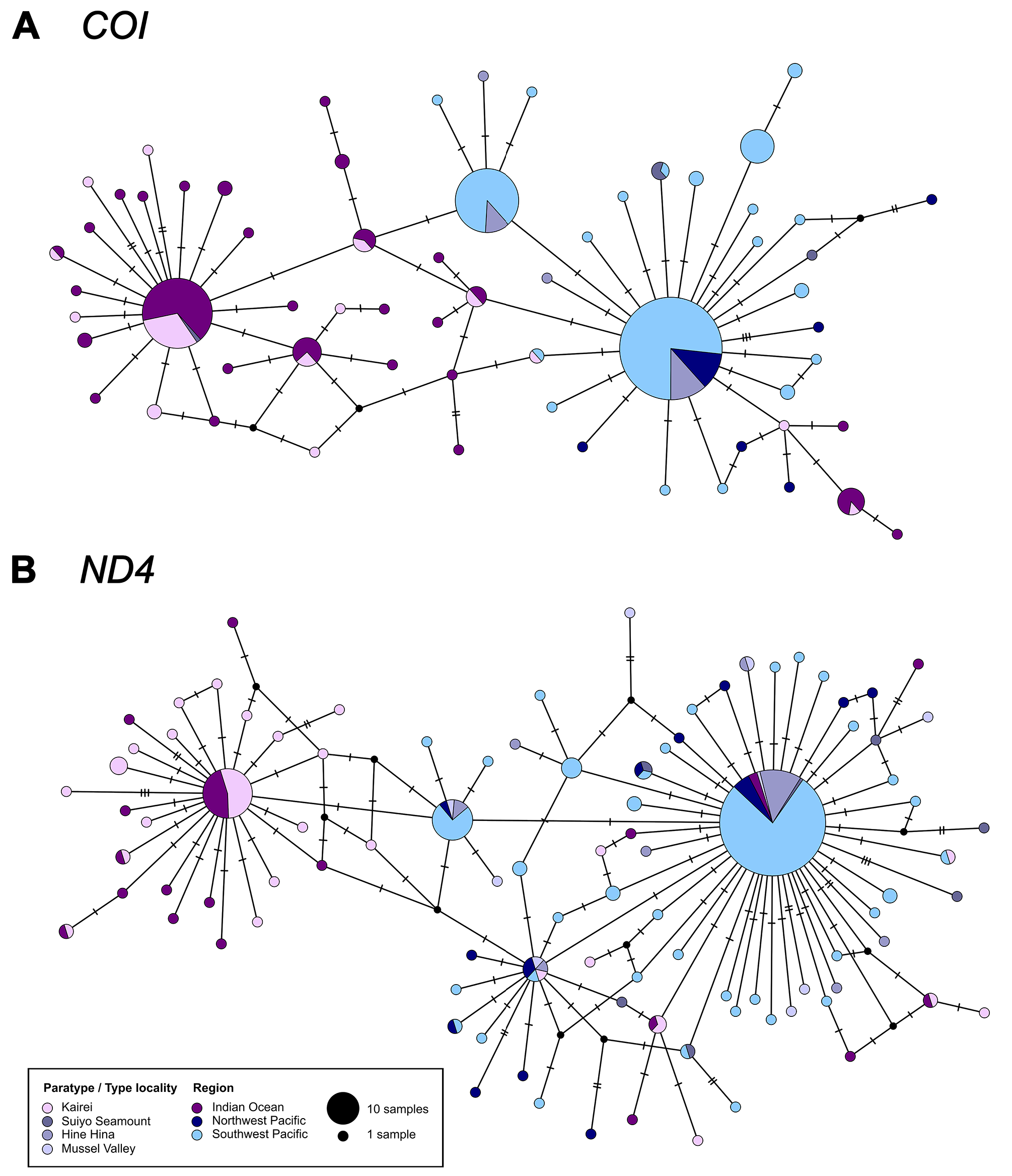
Remarks. Given both the phenotypic variability and the wide geographic spread, it is not surprising that separate species were described from three ocean regions. Hashimoto (2001) provides a table of distinguishing characters among these species; however, we find that the noted shell differences are encompassed by larger samples from both NW and SW Pacific; relative position of the beak is one example. Within samples from one site, shell and scar characters may also vary as noted by Cosel et al. (1994) for ratio of height to length.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.