Tormus Sharp, 1884
|
publication ID |
https://doi.org/ 10.5281/zenodo.4503766 |
|
DOI |
https://doi.org/10.5281/zenodo.4597005 |
|
persistent identifier |
https://treatment.plazi.org/id/03FA580C-852F-FF95-6BB3-FC01FE4AC643 |
|
treatment provided by |
Felipe |
|
scientific name |
Tormus Sharp, 1884 |
| status |
|
Tormus Sharp, 1884 View in CoL
Tormus Sharp, 1884: 474 View in CoL . Type species: Tormus helmsi Sharp, 1884 View in CoL (by monotypy).
Tormus: BROUN (1893a: 1018 View in CoL , diagnosis); ZAITZEV (1908: 376, catalogue, in Hydrobiina); ORCHYMONT (1919: 106, transferred to Rygmodini View in CoL ); KNISCH (1924: 106, in Rygmodini View in CoL ); HANSEN (1991: 182, redescription, transferred to Tormissini View in CoL ); HANSEN (1997: 359, key to species); HANSEN (1999b: 237, catalogue); LESCHEN et al. (2003: 18, list of New Zealand genera); SHORT & FIKÁČEK (2011: 85, list of genera).
Stygnohydrus Broun, 1893b: 1018 View in CoL . Type species: Stygnohydrus nitidus Broun, 1893b View in CoL (by monotypy).
Stygnohydrus: ZAITZEV (1908: 376 View in CoL , catalogue, in Hydrobiina); ORCHYMONT (1919: 106, transferred to Rygmodini View in CoL ); KNISCH (1924: 106, in Rygmodini View in CoL ); HANSEN (1991: 183, synonymized with Tormus View in CoL ).
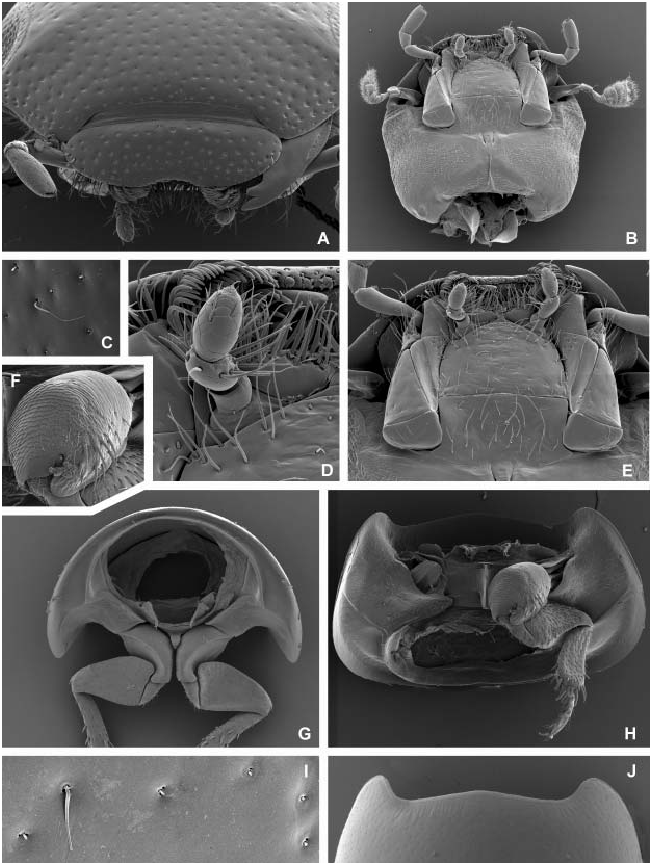
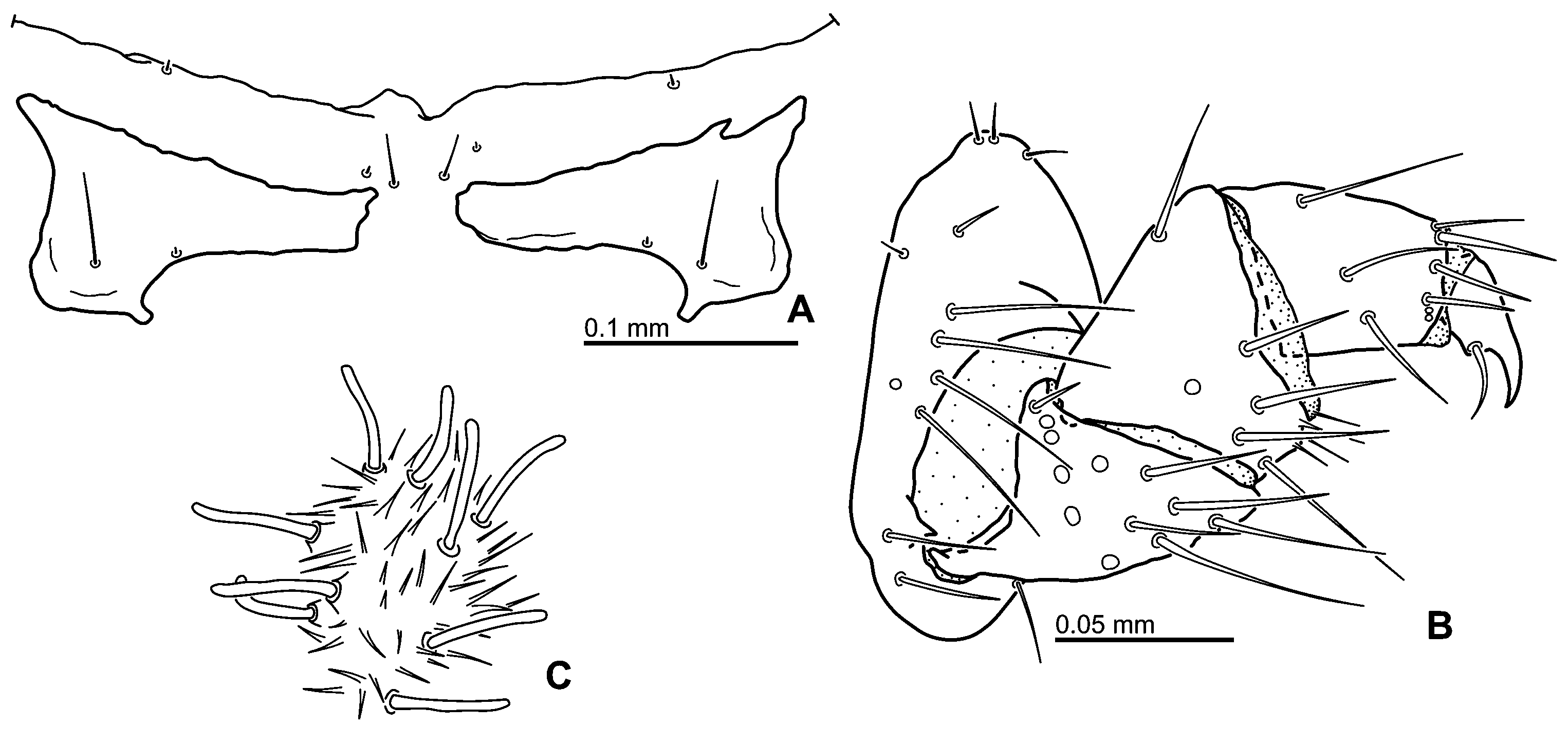
Diagnosis. Adult. Body highly convex, compressed from sides ( Fig. 1A View Fig ); dorsal coloration black, without metallic reflections; clypeus without anterolateral bead ( Fig. 4A View Fig ); clypeus with wide anterior emargination exposing the membrane between clypeus and labrum ( Fig. 4A View Fig ); labrum completely exposed in dorsal view; eyes not protruding; antenna with 9 antennomeres ( Fig. 3F View Fig ); mandibular apex with two apical teeth ( Fig. 3C View Fig ); prosternum extremely short in front of procoxae, bearing longitudinal carina mesally ( Fig. 4H View Fig ); mesoventrite divided from mesanepisterna by distinct anapleural suture ( Fig. 5A View Fig ); anteromedian portion of mesoventrite with a deep pit ( Figs 5 View Fig B–C); posteromedian portion of mesoventrite with narrow high triangular projection ( Fig. 5B View Fig ); elytron with serial punctures present only posterolaterally ( Figs 1A View Fig , 5K View Fig ); coxae each with several strong spines ventrally ( Figs 4F View Fig , 5C, 5E, 5H View Fig ); femora without dense pubescence even basally; basal metatarsomere much shorter than metatarsomere 2 ( Fig. 7C View Fig ); abdomen with five ventrites; basal abdominal ventrite not carinate mesally ( Fig. 6A View Fig ); abdominal laterosternite 3 with organized stridulatory file ( Fig. 6C View Fig ); abdominal apex without apical emargination.
By the dark dorsal coloration, highly convex body and partly reduced elytral series, Tormus resembles some genera of the tribes Chaetarthriini ( Amphiops Erichson, 1843 and Micramphiops Short, 2009 : differs by completely divided eyes; Chaetarthria Stephens, 1835 : differs by the presence of series of long setae over the abdominal ventrite 1; Guyanobius Spangler, 1986 : differ by concealed labrum and femora with dense pubescence basally), Berosini (they differ by 8-segmented antennae and abdominal sternite 7 which is concealed within abdomen or visible as fifth abdominal ventrite with a semicircular or rectangular emargination), Anacaenini (some Anacaena Thomson, 1859 : differ by prosternum without median carina and femora with basal dense pubescence) and Coelostomatini (differ in entire clypeus). It may be easily distinguished from all other New Zealand hydrophilids by the combination of highly convex and laterally compressed body, black dorsal coloration, prosternum very short in front of procoxae and carinate mesally, 9-segmented antennae and abdominal ventrite 1 ecarinate mesally.
Larva. Head ca. as long as wide ( Fig. 14A View Fig ), stemmata very distinct and clearly divided from each other ( Fig. 10A View Fig ); nasale slightly asymmetrical, bearing three teeth of which two right ones are smaller than the left one ( Figs 10C View Fig , 14B View Fig ); epistomal lobes reaching ca. as far as nasale, nearly symmetrical; frontal lines reaching posterior margin of head ( Fig. 10A View Fig , 14A View Fig ), coronal line absent; submentum clearly divided from head capsule; mandibles symmetrical, each with three inner teeth ( Figs 11 View Fig C–D, 13C–D, 15C–D); mesal portions of antennae, maxillae and dorsal face of labium with dense and long pubescence ( Figs 11 View Fig , 13 View Fig , 15 View Fig ); antennae short and stout; antennal sensorium as long as antennomere 3 (e.g., Figs 15 View Fig A–B); stipes with five strong setae ( MX 7–11) on inner face ( Fig. 11E View Fig ); maxillary palpus ca. as long as stipes; inner appendage of maxillary palpomere 1 present and well sclerotized; ligula absent ( Figs 11 View Fig G–I, 13G–I, 15G–I); proscutum without bulges or protruding lobes ( Figs 1 View Fig B–D); anterior margin of proscutum with dense row of setae ( Fig. 14C View Fig ); meso- and metathorax with small tergites only ( Fig. 14F View Fig ); prosternite in form of a pair of separated transverse sclerites ( Figs 12A View Fig , 14E View Fig ); legs 5-segmented but short ( Fig. 12B View Fig ), barely visible in dorsal view; abdomen with indistinct lateral but quite distinct dorsal lobes ( Figs 1 View Fig B–D); median and lateral lobes of spiracular atrium fused into one lobe, bifurcate.
The larvae of Tormus are most similar to those of Paracymus (based in mandibles with three inner teeth, antennal sensorium as long as antennomere 3, frontal lines reaching posterior margin of the head, rather long maxillary palpomere 1, slightly asymmetrical nasale with distinct large teeth and rather large nearly symmetrical epistomal lobes), but differ from them by the absence of ligula, densely pubescent mouthparts and nasale with three teeth only. By the combination of the reduced ligula and frontal sulci reaching posterior margin of the head, Tormus may also resemble the larvae of Berosus Leach, 1817 , Laccobius Erichson, 1837 and Oocyclus Sharp, 1882 (all with asymmetrical mandibles and left mandible with specialized comb-like structures and with highly projecting left epistomal lobe bearing a series of many stout setae on anterior margin, Berosus with tracheal gills on lateral sides of abdomen and reduced stigmatic atrium, and Laccobius and Oocyclus with nasale not bearing large distinct teeth) and some groups of the Sphaeridiinae (which differ by nasale without distinct teeth and mandibles always with less than three teeth).
Redescription of adult. Body elongate oval, highly convex, compressed from sides ( Figs 1A View Fig ). General coloration of dorsal surface piceous brown to black, margins of pronotum and lateral margins and apices of elytra pale reddish to various extent; ventral parts of prothorax brownish, meso- and metathorax blackish, epipleura pale reddish; head appendages reddish; legs with dark reddish brown coxae, femora and tibiae; tarsomeres yellowish.
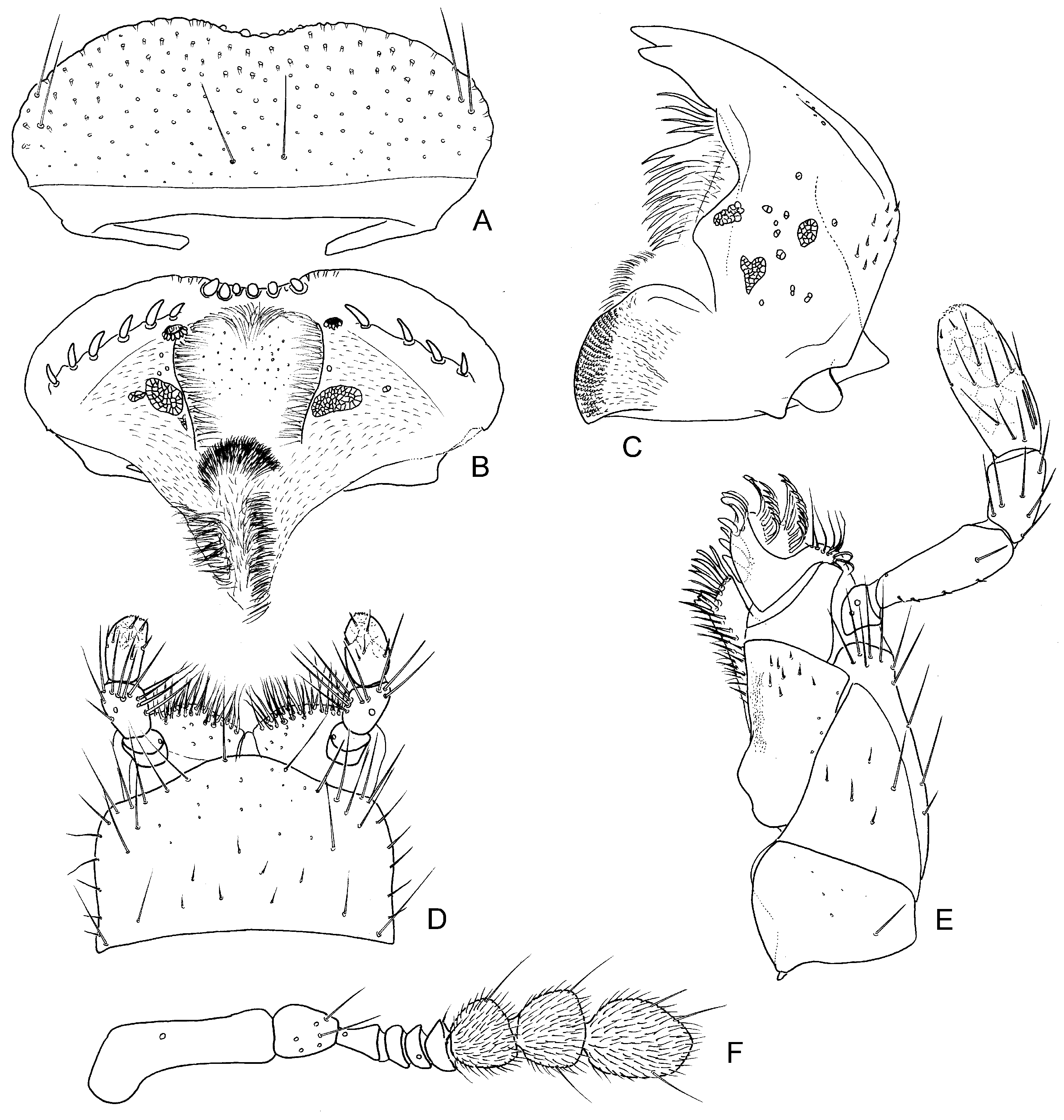
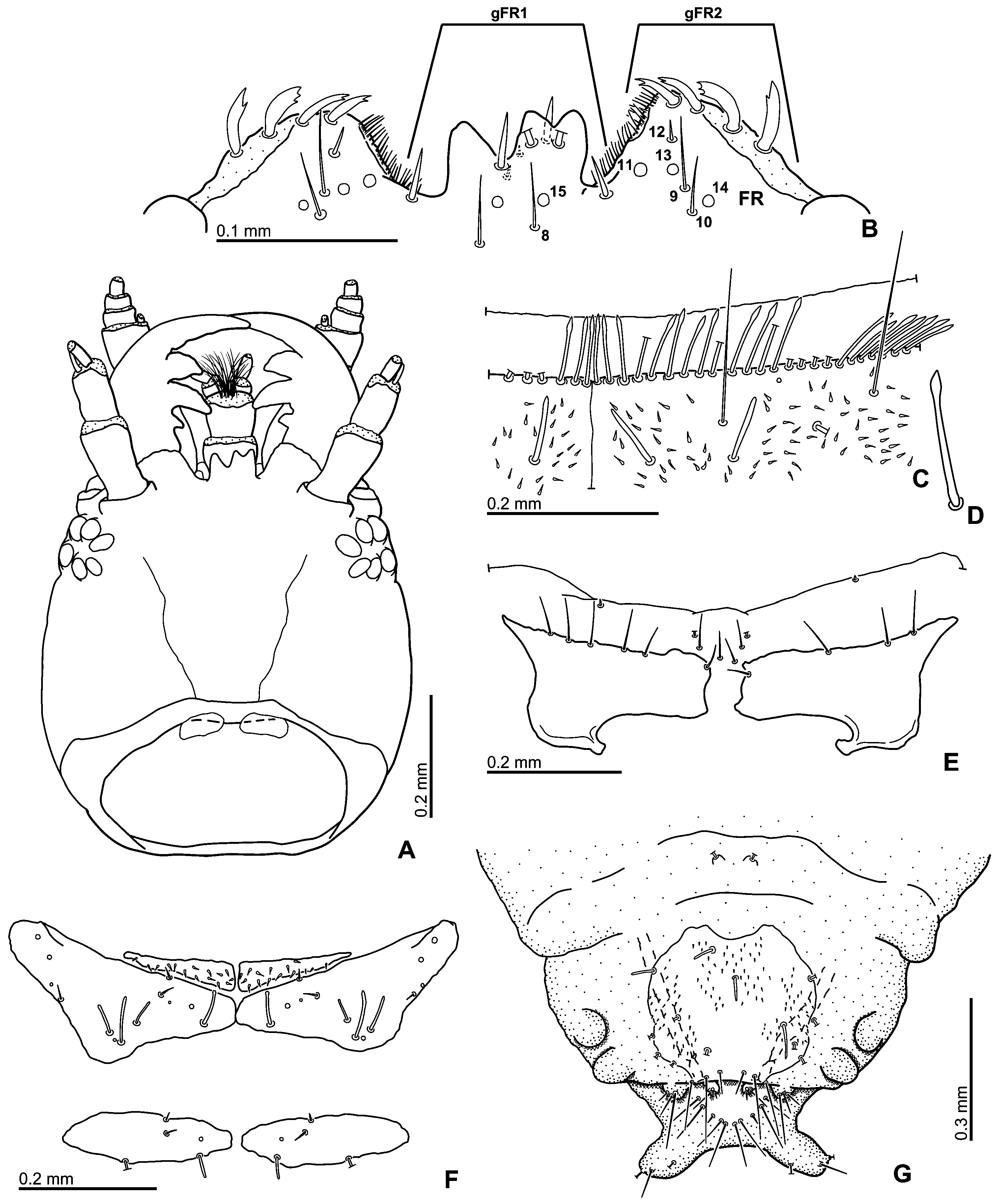
Head. Clypeus and frons ( Fig. 4A View Fig ) distinctly punctate, frons with several trichobothria ( Fig. 4C View Fig ), clypeus without trichobothria; frontoclypeal suture very indistinct; clypeus slightly expanded laterally, covering bases of antennae, anteromedian margin deeply excised, exposing the membrane between clypeus and labrum, lateral margins of clypeus without a bead. Eyes small, not protruding from outline of head, separated by 4.5× width of one eye. Labrum ( Figs 3 View Fig A–B) well sclerotized, completely exposed dorsally, widest subbasally, strongly narrowed basally and arcuately narrowing anteriad, shallowly bisinuate on anterior margin; dorsal surface bearing two pairs of long sublateral setae and one pair of submesal setae and the ground punctation similar to that on clypeus; anterior margin mesally with a series of stout blunt spines; epipharynx with a lateral row of stout large spines on each side, median portion with two vertical rows of long cuticular spines, submesally with one pair of anterior and one pair of large posterior porose fields, basal portion with densely pubescent membranous cone. Mandibles ( Fig. 3C View Fig ) symmetrical, with arcuate lateral margin, bifid mandibular apex; mediodistal portion with a series of long cuticular projections, medioproximal portion with very fine setae, mola rather large. Maxilla ( Fig. 3E View Fig ) with a large subtriangular cardo lacking trichobothria; basistipes triangular, bearing few fine setae only; mediostipes well delimited from lacinia, lacinia moderately sclerotized, bearing stout spines and long thin cuticular projections distally and finer hair-like setae mesally; galea short, with rather stout distal setae arranged into well-defined rows; palpifer rather small, with few rather long setae; maxillary palpus with 4 palpomeres, palpomere 1 minute, palpomeres 2 and 4 subequal in length, ca. twice length of palpomere 3; base of palpomere 4 with a group of ca. three digitiform sensilla on lateral surface. Labium ( Fig. 3D View Fig ) with submentum ca. as long and wide as mentum, bearing sparsely arranged setae; mentum transverse, ca. 1.7× wider than long, with continually convex anterior margin and subparallel lateral margins, its surface bearing numerous setae along anterior margin, lateral margins with few sparsely arranged setae only; prementum subdivided into two membranous lobes bearing anteromedian group of long setae, palpifer vaguely sclerotized; labial palpus with three palpomeres, palpomere 1 minute, palpomere 2 ca. as long as palpomere 3 in both sexes, unmodified in males, bearing numerous rather long and stout setae; palpomere 3 without digitiform sensilla, bearing few minute sensilla. Antenna ( Fig. 3F View Fig ) with 9 antennomeres, scapus conical, ca. 3× as long as pedicel, pedicel widest proximally, bearing a few pore-like sensilla and two tiny setae, antennomere 3 ca. as long as antennomeres 4–6 combined, cupula small and simple, antennomeres 7–9 forming a distinct, loosely segmented and densely pubescent antennal club. Gula ( Fig. 4B View Fig ) extremely narrow, gular sutures nearly fused posteriorly of tentorial pits, the latter closely aggregated, distinct, elongate. Temporae without distinct ridge arising from inner margin of each eye.
Prothorax. Pronotum ( Figs 1A View Fig , 4 View Fig I–J) highly convex, widest subbasally, bearing weakly projecting anterior corners; surface smooth, without any depressions, with distinct punctation, few trichobothria present anterolaterally and posterolaterally; anterior margin slightly angulate mesally, lateral margins forming continuous curve with posterior margin; lateral margin arcuately bent to posterior margin; marginal bead present along whole anterior and lateral margins. Hypomeron ( Fig. 4H View Fig ) with rather narrow lateral glabrous portion and densely pubescent median portion, portions not divided by a ridge, hypomeral process large, bearing wide marginal bead, arcuately pointed mesally. Prosternum ( Fig. 4H View Fig ) extremely short anterior to procoxae, ca. 0.1× as long as procoxa; mesal portion largely concealed by proxocae but strongly carinate mesally, prosternal process large, nearly completely concealed by procoxae. Coxal cavities delimited internally by median prosternal carina, open posteriorly, coxal fissure rather long, open, notopleural suture distinct but very short. Accessory ridge below posterior pronotal margin very distinct, laterally reaching to lateral margin of hypomeron as a ‘transverse fold’. Profurca ( Fig. 4G View Fig ) very short, profurcal arms widely separated, in the form of short but large slightly asymmetrical plate-like extensions directed posteriad and slightly extending of the prothoracic cavity.
Mesothorax. Scutum ( Fig. 5G View Fig ) bearing few setae mesally; scutellar shield exposed, triangular, pointed posteriorly, ca. as long as wide, bearing indistinct colon-like punctures on its surface. Elytron ( Figs 1A View Fig , 5F, 5 View Fig I–K) highly convex; sutural stria present, reaching ca. midlength of elytron; nine elytral series distinct only posterolaterally, obliterated anteromesally, formed by punctures of the same size but slightly more impressed than interval punctation; scutellary stria absent (not visible even in slide-mounted elytron); alternate elytral intervals each with numerous trichobothria, punctures of elytral intervals colon-like, each bearing a short club-like seta; lateral edge with a narrow bead; epipleuron moderately wide anteriorly, gradually narrowing to elytra midlength, indistinct more posteriad, its median pubescent portion delimited from lateral narrow bare one by a straight fine line or ridge; ventral elytral surface without any elevated ridges, only with a narrow longitudinal field of fine spines situated sublaterally between anterior fourth and midlength. Mesoventrite ( Figs 5 View Fig A–C) divided from mesanepisternum by distinct anapleural suture; mesoventrite subtriangular in shape, widely extending laterad posteriorly, lateral extensions bearing distinct coxal lobes; median portion of mesoventrite elevated into a narrow median crest, the crest strongly angulate in lateral view, bearing few stiff setae apically; mesoventral process narrow; anteromesal portion of mesoventrite with a deep pit; whole surface with scale-like microsculpture, mesally bearing sparsely arranged setae; trochantin well divided from mesoventrite, transverse, long and narrow. Mesanepisterna not meeting anteromesally, narrowly divided by anterior portion of mesoventrite; anterior collar well-defined, moderately wide; mesal portion of each mesanepisternum with few setae, large lateral portions bare. Mesepimeron with large ventral portion, not reaching anterior collar of mesanepisternum anteriorly, forming lateral margin of coxal cavity; its surface with few setae only. Coxal cavities obliquely transverse, ca. 3× wider than long, very narrowly separated from each other by mesoventral and metaventral processes; internal postcoxal wall moderately wide mesally and posteriorly. Mesofurca ( Fig. 5D View Fig ) well-developed, long, arising as two basally fused arms from posterior wall of coxal cavities, bearing plate-like extensions at midlength and reaching to dorsolateral body walls.
Metathorax. Metanotum very short, weakly sclerotized, ca. 5.5× wider than long, with nearly totally reduced anterior membranous area, alacristae widely separated, slightly diverging anteriad. Metaventrite ( Fig. 5A View Fig ) short, ca. as long as mesoventrite, evenly convex, with slightly elevated median portion well defined posteriorly by a blunt ridge with slightly stouter setae, whole surface (except for a small posterior area) bearing rather dense pubescence; metacoxal process rather long and distinctly exposed. Postcoxal ridge very narrow but well-defined. Metanepisternum ca. 7.5× longer than wide, without an obliquely transverse strengthened ridge anteriorly; whole surface sparsely pubescent. Metepimeron without distinct ventral portion. Metafurca ( Figs 7D View Fig ) rather large, Y-shaped; stalk very short, without basal extensions; lateral arms rather long, without anterobasal extensions, apical portions widened. Hind wing absent.
Legs ( Figs 7 View Fig A–C). Coxae with numerous (procoxae) or few (meso- and metacoxae) large spines ventrally close to trochanter articulation, otherwise bare; procoxae subglobular, slightly transverse; mesocoxae transverse, rather robust mesally, narrowly separated; metacoxae narrowly transverse, subrectangular in ventral view. Trochanters with proximal parts concealed by coxae, distal subtriangular parts exposed ventrally, bearing sparsely arranged setae. Femora attached to trochanters by their posteromesal (in meso- and metafemora) or anteromesal (on profemora) portions only, anteromesal (in meso- and metafemora) or posteromesal bases (in profemora) free, rounded; profemora sparsely pubescent ventrally (except for bare posterobasal portion), meso- and metafemora with short sparsely arranged spines; tibial grooves present, deep, defined by a high ridge ventrally and low ridge dorsally. Tibiae slightly longer than femora, abruptly widening subproximally, slightly widening distad; each tibia with three dorsal, three outer lateral, and irregular ventral and inner lateral series of spines; ventral, outer lateral and distally situated inner lateral spines stout and long. Tarsi with 5 tarsomeres, basal pro- and mesotarsomere subequal in length to tarsomere 2, basal metatarsomere distinctly shorter than metatarsomere 2; pro- and mesotarsomere 5 nearly as long as tarsomeres 3–4 combined, metatarsomeres 4 and 5 subequal in length. All tarsomeres with numerous moderately long setae ventrally and few setae of same length dorsally; claws arcuate, without subbasal tooth; tarsi and claws not sexually dimorphic.
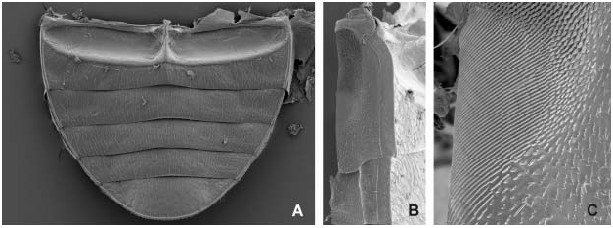
Abdomen ( Figs 6 View Fig A–B) with five exposed ventrites; ventrite 1 with moderately large bare coxal grooves, remaining portion sparsely pubescent, median portion without longitudinal carina; ventrites 2–4 subequal in length, sparsely pubescent on whole surface; ventrite 5 slightly longer than preceding ones, its posterior margin without median emargination or group of enlarged setae; lateral margins of ventrites 1–4 and lateral and posterior margin of ventrite 5 with narrow but distinct bead. Laterosternite 3 simple, dorsal portion not divided from ventral by a ridge, bearing an area of goose-head-shaped cuticular projections which is posteriorly organized into a stridulatory file consisting of obliquely arranged uninterrupted lamellae; laterosternites 4–6 step-like, subdivided into elevated ventral and depressed dorsal portion, nearly without cuticular projections; tergites 1–6 membranous, bearing very fine cuticular asperities posteriorly, tergite 7 moderately sclerotized.
Genitalia. Male genitalia ( Figs 7F View Fig , 9 View Fig ). Aedeagus of simply trilobed type; parameres ca. as long as phallobase, wide basally, narrowing apicad, apices species-specific in shape; whole paramere bearing numerous pore-like sensilla; median lobe slightly shorter than parameres, widely subrectangular in apical portion, slightly angulate on apex, apodemes rather long and reaching into phallobase, gonoporus apical; phallobase with moderately large but rather indistinctly detached manubrium. Sternite 9 widely tongue-like, with long subbasal lateral struts. Sternite 8 crescent-like without distinct anterior projection. Female genitalia ( Fig. 7E View Fig ). Coxostyli 9 very long, cylindrical, gonostyli 9 very short, ca. 0.1× as long as coxostyli 9; each mediotergite 8 with two setae and two pores posteriorly.
Description of larva. See under Tormus helmsi .
Biology. Both adults and larvae are terrestrial, inhabiting moss on the ground and on fallen decaying trunks ( Figs 2 View Fig C–D) in forests (e.g., Figs 2 View Fig A–B) or closely above tree line in the lower alpine zone. Most specimens collected by us were found by sifting moss, less frequently singletons were found in samples of forest leaf litter. Infrequently, adults were also found on the underside of wet rotting logs on forest floor, especially when these are lying next or on the moss ( Fig. 2E View Fig ). Label data also indicate that adults are sometimes found on moss and tree trunks at night. Immature stages were collected together with adults in all known cases, the larvae have three larval instars, typical for the Hydrophilidae .
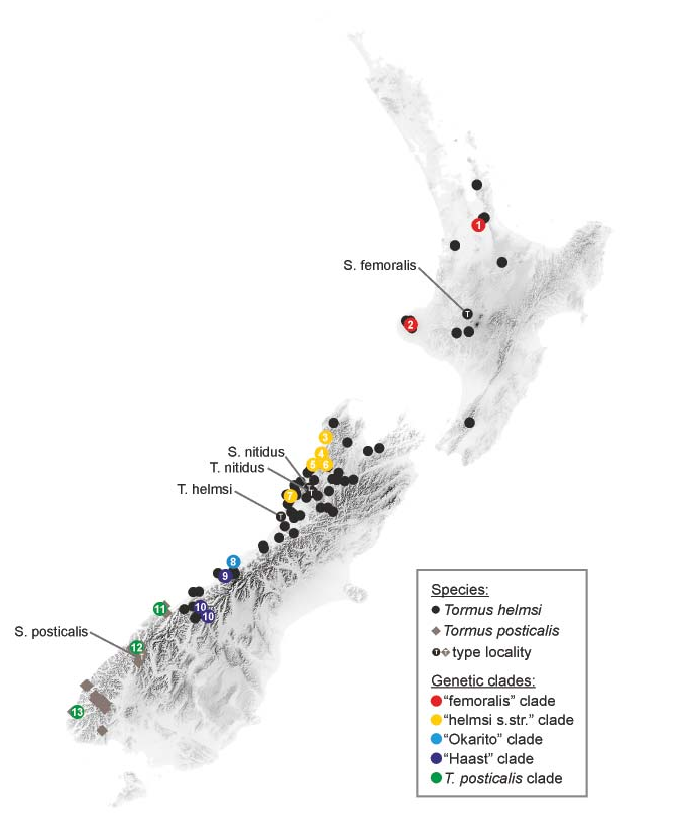
Distribution. The genus is endemic to the two main islands of New Zealand ( Fig. 16 View Fig ).
Status of Stygnohydrus . BROUN (1893b) erected Stygnohydrus based on a single teneral specimen (type of S. nitidus Broun, 1893 ) and mentioned that it is closely related to Tormus which he knew from SHARP’ s (1884) original description. BROUN (1893b) mentions the ecarinate prosternum and the carinate abdominal ventrite 1 in the description of S. nitidus (type species of Stygnohydrus ), which are characters he likely considered diagnostic of Tormus . Both characters were apparently misinterpreted by BROUN (1893b, 1910, 1917) as he overlooked the prosternal carina concealed by the procoxae and considered the ridge between the metacoxal grooves of abdominal ventrite 1 as a median carina (see also HANSEN 1991: 183). All species of Tormus described subsequently by BROUN (1893c, 1910, 1917) were already found congeneric by Broun himself, i.e. described as Stygnohydrus or transferred there after the description (see the note about the letter of Broun to d’Orchymont in ORCHYMONT (1937: 155)). As Broun never examined the types of Tormus helmsi , he did not realize they were congeneric with Stygnohydrus and that the supposed differences in prosternal and abdominal morphology do not exist. For that reason, Tormus and Stygnohydrus were maintained as separate genera until HANSEN (1991) compared their type species.
Genetic and morphological diversity. Each locality sampled exhibited unique haplotype, which may be related to the small sample sizes sequenced. The only exception is Mt. Te Aroha where one larva and one adult, both from the same sample, were sequenced, and shared the same haplotype (also, confirming the identity of the larvae used for the morphological description below).
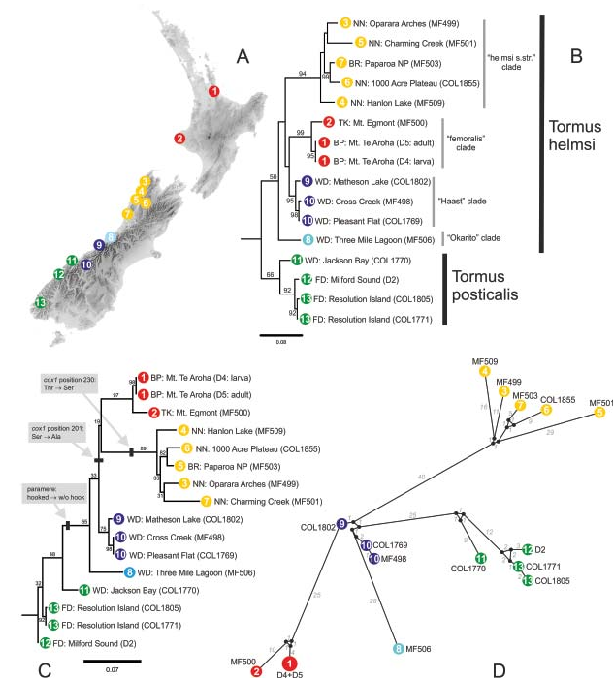
TIM2 with invariable sites (+I) and rate variation among sites (+G) following the gamma distribution was selected as the optimal nucleotide substitution model for our cox1 data under the Aikake and corrected Aikake information criterion as implemented in jModeltest. Bayesian analysis recognized five monophyletic clades which are geographically limited and allopatric ( Fig. 8B View Fig ), the same groups were also recognized as distinct clusters in the haplotype network analysis ( Fig. 8D View Fig ), resulting in the network with the ‘Haast’ group in the centre. Maximum likelihood analysis also recognized the same groups as other analyses ( Fig. 8C View Fig ) but placed the root of the tree (i.e. Paracymus pygmaeus used as an outgroup) within the southernmost (green) cluster, making it therefore paraphyletic. The overall generic diversity of Tormus was found very high (6.5 %) with the mean divergences ranging between 0.5–3.9 % within each group, and between 4.2–9.0 % between groups. The highest within-group diversity was found in the yellow (‘ helmsi s. str. ’) clade from northern South Island which was however represented by the highest number of specimens (n = 5). Two conserved groupspecific amino acid changes were found ( Fig. 8C View Fig ): Ser-Ala at position 201 (alanine in red and yellow groups, serine in remaining groups of Tormus ) and Thr-Ser at position 230 (serine in the yellow group, threonine in all remaining Tormus groups). The Ser-Ala change indicates that the sister relationship of the red (‘ femoralis ’) and yellow (‘ helmsi s. str. ’) clades as recognized by the ML analysis is more likely than the topology revealed by the Bayesian analysis.
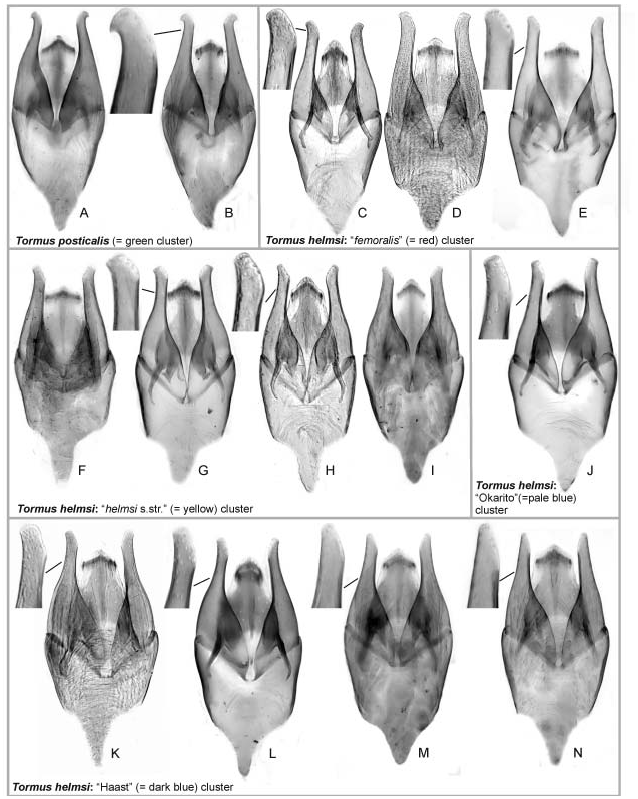
All examined specimens of Tormus are very uniform in morphology, with variation only detected in the male genitalia (shape of the paramere apex), strength of dorsal punctuation of elytra, and the declivity of the posterior portion of elytra in lateral view. The highest diversity of aedeagal morphology was found in the specimens from central and southern Westland (‘Haast’ clade) which genitalia resemble those from the North Island and northern South Island, plus contain the morphotypes without widened apex of parameres unique for the clade. Specimens from northern South Island are also quite variable with aedeagi resembling those of North Island and ‘Okarito’ groups, but without any unique morphotypes. The North Island populations have all rather uniform and only slightly variable aedeagus. The same is true for the southern South Island clade, in which only a slight intraspecific variation was observed and which are easily distinguishable from remaining clades by the parameral hooks. Dorsal punctuation of anterior portion of the elytra and the declivity of elytra in lateral view are rather uniform only in the North Island specimens (with coarse elytral punctation and steep posterior portion of elytra) whereas the South Island ones vary in these characters (elytral punctuation very fine to strong, posterior portion of elytra oblique to nearly vertical). The variation of the non-genital characters is continuous and was unreliable for sorting material into distinct morphotypes.
Species-level taxonomy. The morphological study revealed that the species-level taxonomy of Tormus is a complex issue. Five species were originally described, of which only three were considered valid based on study of the type material by HANSEN (1997). The characters HANSEN (1997) used for delimiting species (coloration and strength of the dorsal punctation) were unreliable to distinguish specimens with clear differences in aedeagal morphology and are moreover slightly variable in specimens from the same locality. No additional external characters corresponding with the differences of genital morphology were found even by a detailed study using SEM. A study of genital morphology of specimens from all localities from which males were available showed three facts: (1) the Fiordland and southernmost Westland specimens have a very distinct aedeagus with hooks on paramere apices and vary only very slightly in genital morphology; (2) the specimens from North Island all have rather uniformly shaped genitalia, but the same genital morphology is also found in some South Island specimens; and (3) the specimens from northern half of South Island (Nelson, Buller and central and nothern Westland) have very variable genitalia and no clear correlation with geography may be observed. Morphology was therefore found as insufficient to resolve species-level taxonomy of Tormus , assuming that multiple species exist, which is the reason why the sequence data of the cox1 fragment were examined.
Although the precise pattern of the basal branching was in conflict between Bayesian and ML analyses and the support of the clades was low in both cases, both analyses agree in reconstructing the southernmost (green) cluster on the base of the tree and grouping the remaining groups into a weakly supported but monophyletic clade. This division is in agreement to morphology of the aedeagus, namely with the presence (in green group) or absence (in remaining groups) of the apical hook of the paramere. The green group, therfore, fulfills traditional criteria of species delimitation and can unambiguously be identified as a separate species, T. posticalis .
Representatives of the remaining clusters show deep genetic divergences correlated with geography but cannot be reliably recognized by means of genital morphology. Still, their distances to the closest group vary between 4.7–7.5 % that corresponds or is even higher than minimum distance delimiting T. posticalis (i.e. the only clade which separate species status is unambiguous). The dark blue (‘Haast’), pale blue (‘Okarito’) and red (‘ femoralis ’) clades are moreover genetically more similar to T. posticalis than to the yellow (‘ helmsi s. str. ’) clade. Combined with the reciprocal allopatry of all clades these results indicate that the four clades may represent four separate geographically limited cryptic species or distinct populations of a single variable species, but additional genetic studies are required. For practical taxonomic purposes, we distinguish two morphologically distinct taxa below: Tormus posticalis characterized by the hook-like apices of parameres, and T. helmsi (understood here as a complex of four haplotype lineages) characterized by the absence of the parameral hooks.
Full Mantel tests for isolation by distance showed weak to moderate correlation between genetic and geographic distance for complete data as well as for populations of T. helmsi . Partial Mantel tests show moderate partial correlation between genetic distance and cluster membership controlled for geography in both cases, and very weak or no correlation between genetics and geography when controlled for cluster membership. These results ( Table 4 View Table 4 ) indicate that isolation by distance does not explain the observed genetic variation of Tormus and that some environmental (or geographic) barriers limiting dispersal are, or were, present to structure current populations.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Tormus Sharp, 1884
| Fikáček, Martin, Minoshima, Yûsuke, Vondráček, Dominik, Gunter, Nicole & Leschen, Richard A. B. 2013 |
Stygnohydrus: ZAITZEV (1908: 376
| HANSEN M. 1991: 183 |
| KNISCH A. 1924: 106 |
| ORCHYMONT A. 1919: 106 |
| ZAITZEV F. A. 1908: 376 |
Tormus: BROUN (1893a: 1018
| SHORT A. E. Z. & FIKACEK M. 2011: 85 |
| LESCHEN R. A. B. & LAWRENCE J. F. & KUSCHEL G. & THORPE S. & WANG Q. 2003: 18 |
| HANSEN M. 1999: 237 |
| HANSEN M. 1997: 359 |
| HANSEN M. 1991: 182 |
| KNISCH A. 1924: 106 |
| ORCHYMONT A. 1919: 106 |
| ZAITZEV F. A. 1908: 376 |
| BROUN T. 1893: 1018 |
Stygnohydrus
| BROUN T. 1893: 1018 |
Tormus
| SHARP D. 1884: 474 |