Rhinoxenus cachorra Soares and Domingues, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4700.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:C10998AB-822A-4B17-8FE1-596DC44C7D6A |
|
DOI |
https://doi.org/10.5281/zenodo.5614566 |
|
persistent identifier |
https://treatment.plazi.org/id/9B20D7DD-0B6F-4191-967A-75466A0FCA90 |
|
taxon LSID |
lsid:zoobank.org:act:9B20D7DD-0B6F-4191-967A-75466A0FCA90 |
|
treatment provided by |
Plazi |
|
scientific name |
Rhinoxenus cachorra Soares and Domingues |
| status |
sp. nov. |
Rhinoxenus cachorra Soares and Domingues View in CoL n. sp.
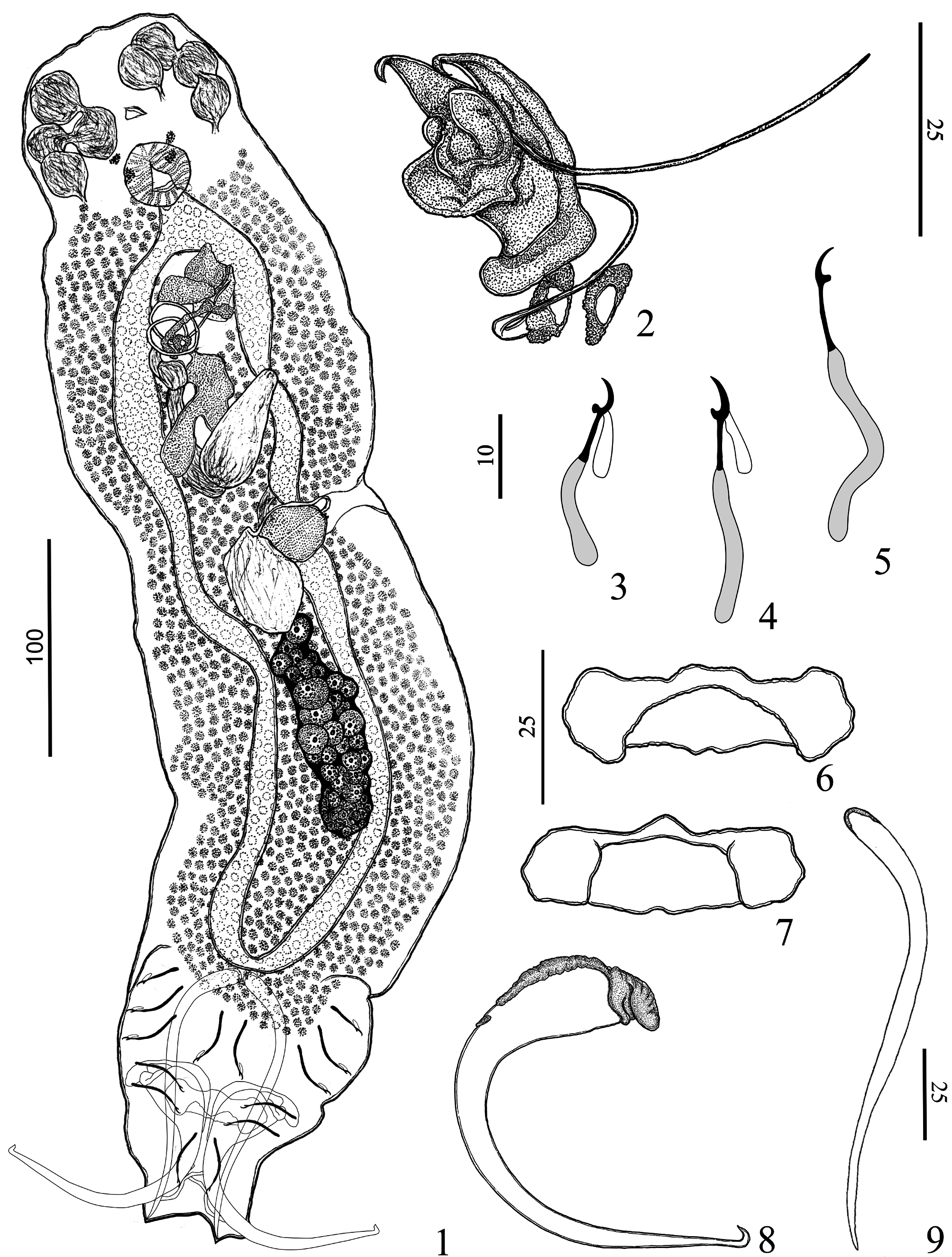
( Figs. 1–9 View FIGURES 1–9 )
Type host: Hydrolycus armatus (Jardine & Schomburgk) .
Site: Nasal cavities.
Type locality: Volta Grande , Xingu River, municipality of Altamira, Pará State, Brazil ( 03°21’15,7’’S; 52°11’47,5’’W), collected on June 13, 2015 GoogleMaps .
Prevalence: 100% of three hosts examined.
Mean intensity: 4.5 parasites per infected host.
Specimens deposited: Holotype, MPEG nº 168; 9 paratypes, MPEG n° 169–177; 3vouchers, MPEG n° 178– 180.
Etymology: The specific name is derived from the common name of the host, “cachorra,” used by the people of the Northeast Amazon, Brazil.
Zoobank Life Science Identifier: (LSID) for Rhinoxenus cachorra n. sp. is urn:lsid:zoobank.org:act:
Comparative measurements: see Table 2 View TABLE 2 .
Description (based on ten specimens; five mounted in Hoyer and five mounted in Gomori’s trichrome): Body fusiform, total length excluding haptor 511 (475–550; n= 5), total width at level of germarium 141 (105–170; n=5) ( Fig. 1 View FIGURES 1–9 ). Tegument smooth. Cephalic region broad; cephalic lobes inconspicuous; three bilateral pairs of head organs with rod-shaped secretion; cephalic glands not observed. Two pairs of eyespots; anterior eyes slightly farther apart than posterior pair; accessory chromatic granules absent. Mouth subterminal, midventral; pharynx muscular, ovate to subspherical, 31 (27–28; n= 6) long, 28 (24–32; n=6) wide; esophagus short. Two intestinal ceca, confluent posteriorly to gonads, lacking diverticula. Common genital pore opening midventral near level of cecal bifurcation; genital atrium muscular. Intercecal gonads, overlapping. Testis dorsal to germarium, fusiform, 37 (25–50; n=2) long, 28 (15–42; n=2) wide (observed only in paratypes). Vas deferens apparently looping left intestinal cecum; seminal vesicle sigmoid, representing a dilation in the vas deferens, with distal portion looping posteriorly before entering MCO base. Single prostatic reservoir, bifurcated, posterior to copulatory complex. Copulatory complex comprising MCO, accessory piece ( Fig. 2 View FIGURES 1–9 ). MCO sclerotized, tubular, spiral, counterclockwise, with two coils, 139 (100–157; n=4) long, base with sclerotized cap, distal aperture acute; circular sclerotized tandem brim associated with MCO base. Accessory piece, articulated with MCO, comprising complex sheath with distal portion expanded, bifid. Germarium fusiform, 52 (40–80; n=4) long, 23 (22–25; n=4) wide. Eggs, Mehlis’ glands, ootype not observed. Vagina single, sclerotized, opening ventrally at the left body margin, at level of vitelline commissure; vaginal vestibule heavily sclerotized at distal portion; vaginal canal sclerotized, sigmoid. Seminal receptacle broad, anterior to germarium. Vitellaria well developed, coextensive with intestinal ceca. Haptor subtrapezoidal, 150 (125–175; n=4) long, 117 (100–137; n=4) wide. Anchors dissimilar. Ventral anchor 111 (107–115; n=5) long, with inconspicuous roots; base 36 (32–38; n=5) width, with sclerotized cap; shaft recurved near mid-length, point with fish-hook-like termination ( Fig. 8 View FIGURES 1–9 ). Dorsal anchor 120 (114–130; n=4), with blunt proximal end covered by subtle sclerotized cap, straight shaft, tapered distal end ( Fig. 9 View FIGURES 1–9 ). Ventral bar with ends ventrally bent toward to its posterior portion ( Figs 6–7 View FIGURES 1–9 ). Dorsal bar absent. Hooks dissimilar in shape, comprising shank of two subunits; filamentous hook loop extended near to beginning of shank dilation; hook pair 2, 28 (25–32; n=10) long, with erect thumb, curved shaft, short point, proximal ½ of shank inflated ( Fig. 3 View FIGURES 1–9 ); hook pairs 1, 3–7, 32 (30–35; n=7) long, with slightly depressed to straight thumb, slightly curved shaft, short point, proximal ¾ of shank inflated ( Figs. 4–5 View FIGURES 1–9 ).
Remarks: Rhinoxenus cachorra n. sp. is similar to Rhinoxenus euryxenus Domingues & Boeger, 2005 mainly due to the morphology of the haptoral structures. Both species have a ventral anchor with inconspicuous roots and a sclerotized cap on the base of the anchor with projection for articulation to ventral bar; shaft recurved near the mid-length, point with fish-hook-like termination; a ventral bar with ends ventrally bent toward to its posterior portion. The new species differs from R. euryxenus by the presence of a spiraled MCO with two counterclockwise coils (from one and a half to two coils in R. euryxenus ), and an accessory piece with an expanded, bifurcated distal portion (cone-like in R. euryxenus ). Finally, these species differ on the morphology of the point of the ventral anchor and hook pair 2; R. euryxenus has the point of ventral anchor with saucer-like termination, and the hook pair 2 is robust, with an erect and robust thumb, whereas Rhinoxenus cachorra n. sp. has the point with hook-like termination, the hook pair 2 is not robust and its thumb, although erect, is also not robust.
| MPEG |
Museu Paraense Emilio Goeldi |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |