Taeniopteryx araneoides Klápalek, 1902
|
publication ID |
https://doi.org/10.11646/zootaxa.4247.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:C736CA96-99A5-40D2-B944-A50410552E37 |
|
DOI |
https://doi.org/10.5281/zenodo.6053065 |
|
persistent identifier |
https://treatment.plazi.org/id/03F887C2-8765-FFBE-FF05-FC1898E8FE10 |
|
treatment provided by |
Plazi |
|
scientific name |
Taeniopteryx araneoides Klápalek, 1902 |
| status |
|
Taeniopteryx araneoides Klápalek, 1902 View in CoL
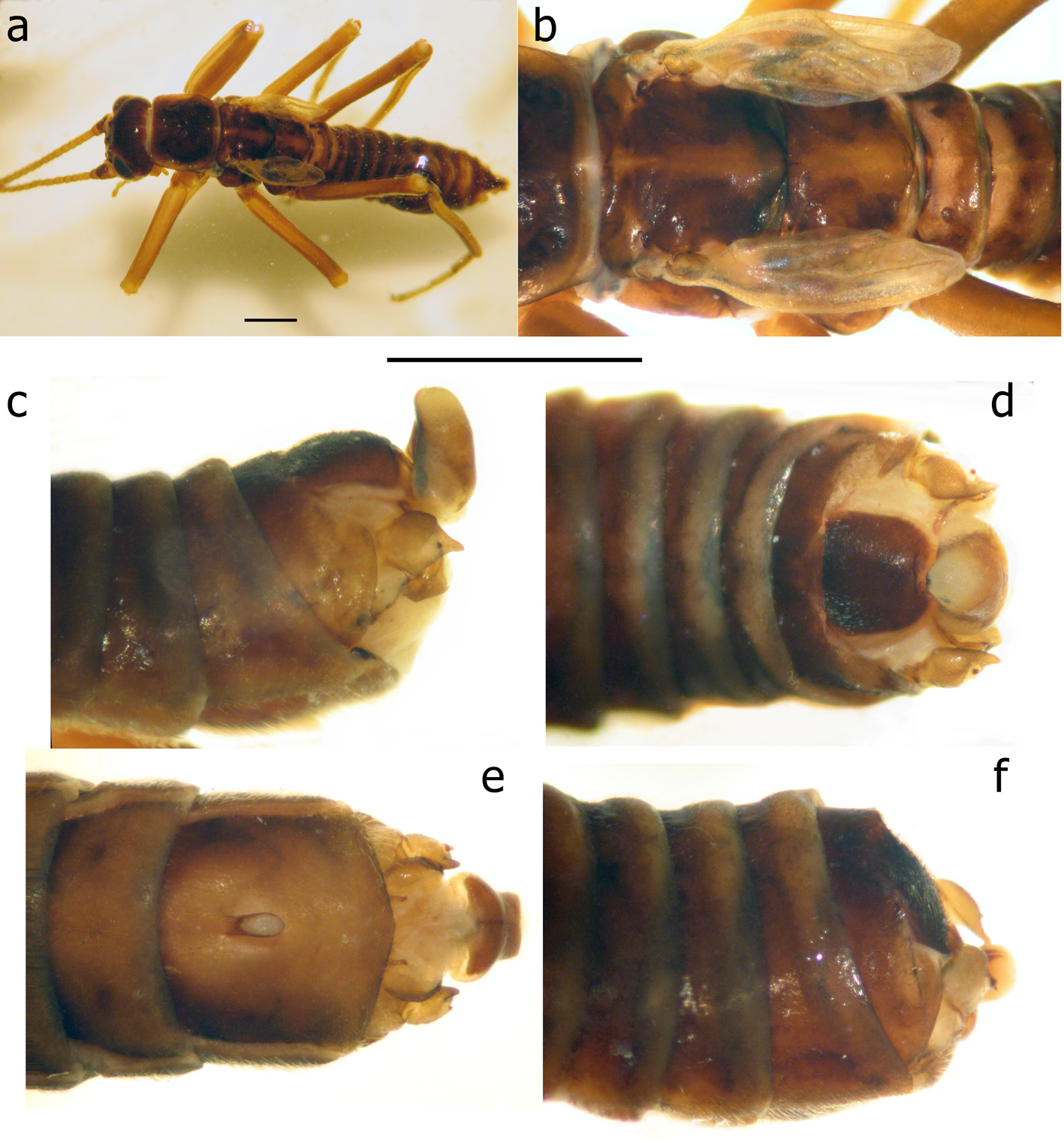
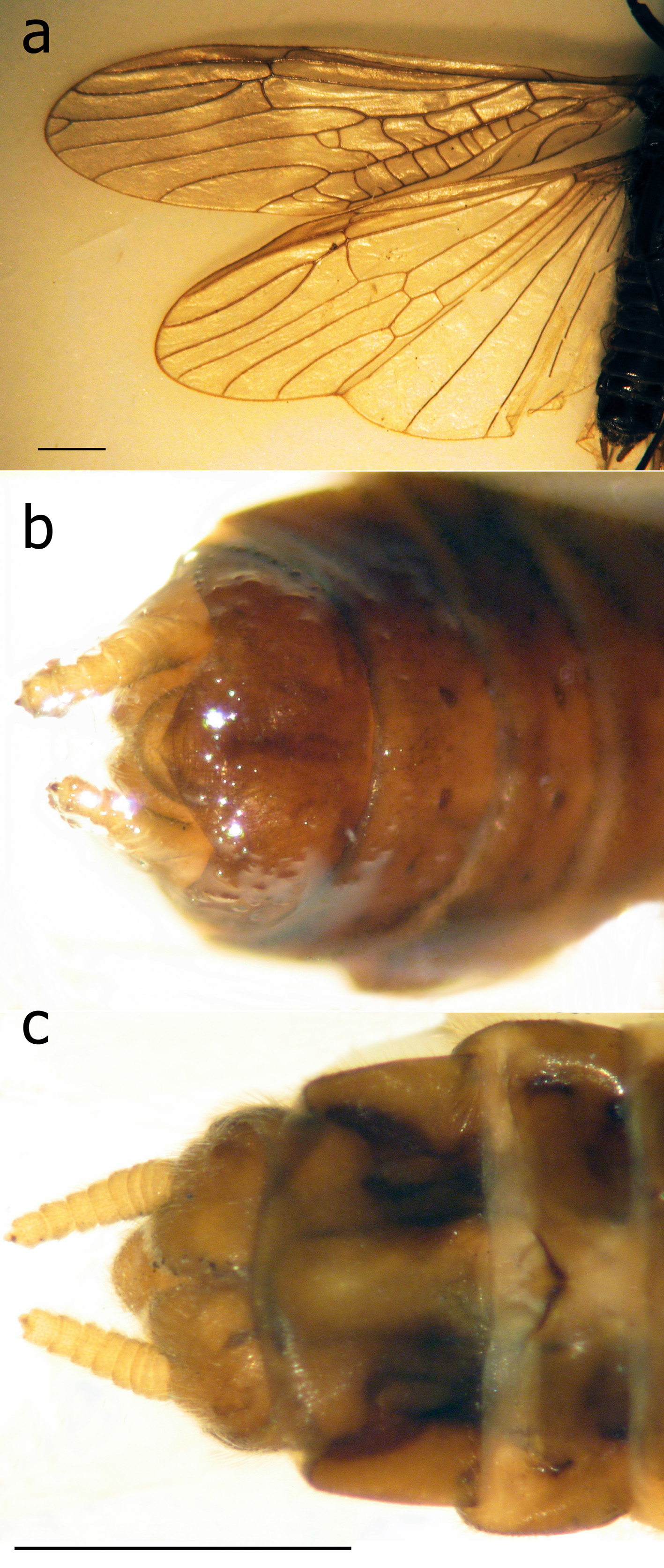
Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3
Reports for Siberia: Zapekina-Dulkeit, 1957: 27–28; Zapekina-Dulkeit, 1971a: 88–90; Zapekina-Dulkeit, 1971b: 223–224.
Specimens studied. Russia, West Siberia , Omsk city centre, the Irtysh River right bank downstream of the Om’ River mouth, 5459' N, 7321–22'' E, 67–68 m a.s.l., O. Kosterin leg.: 34♂ (of them 8 had non-expanded and 3 partly expanded anal appendages), 1♀ in alcohol, 19 ♂, 4 ♀ dry on pins, 2 v 1995 ; 15 ♂ 1♀, the same place, 3 v 2003 (specimens deposited in the Institute of Systematics and Ecology of Animals SB RAS, Novosibirsk, Russia) .
Kazakhstan, Pavlodar Province : 3 ♂, 25 ♀, 20 iv 2014, 5128′ N, 7747′ E, the Irtysh River right bank upstream Pavlodar at Akku (formerly Lebyazhye) village, I. Sivec, N. Akimbekova leg. ; 1 ♂, 2 ♀, the same locality, 20 iv 2015, N. Akimbekova leg. ; 19 ♂, the same locality, 15 iv 2016, N. Akimbekova leg. ; 8 ♂, 97 ♀, 4 exuviae, the same locality, 23 iv 2016, N. Akimbekova leg. ; 23 ♀, the Irtysh River right bank upstream Pavlodar at Dzhambul village, 5130′ N, 7741′ E, 23 iv 2016, N. Akimbekova leg. ; 1 ♂, the Irtysh River right bank upstream Pavlodar at Zhanatan village env., 7 ♀, 5132′ N, 7738′ E, 24 iv 2015, N. Akimbekova leg. ; 2 ♂, 8 ♀, the same locality, 25 iv 2015, N. Akimbekova leg. ; 4 ♂, 22 ♀, 3 exuviae, the Irtysh River right bank upstream Pavlodar at Tlektes village, 5149′ N, 7727′ E, 23 iv 2016, N. Akimbekova leg. ; 37 ♂, 7 ♀, 1 exuvia, the Irtysh River right bank upstream Pavlodar at Novoyamyshevo village, 5155′ N, 7719′ E, 19 iv 2016, N. Akimbekova leg. ; 1 ♂, 5 ♀, 1 exuvia, the same locality, 23 iv 2016, N. Akimbekova leg. ; 19 ♀, the same locality, 6 v 2016, N. Akimbekova leg. ; 30 ♂, 3 ♀, 1 skin, the Irtysh River right bank downstream Pavlodar at Sychovka village, 5236′ N 7645′ E, 21 iv 2016, N. Akimbekova leg. ; 10m #, 82 ♀, the Irtysh River right bank downstream Pavlodar at Naberezhnoe village, 5241′ N, 7643′ E, 22 iv 2015, N. Akimbekova leg. ; 70 ♂, 14 ♀, the same place, 30 iv 2016, N. Akimbekova leg.; 170 ♂, 75 ♀, 7 exuviae, the same place, 16 iv 2016, N. Akimbekova leg.; 1 ♂, 5 ♀, the same place, 24 iv 2016, N. Akimbekova leg.; 8 ♂, 10 ♀, 1 exuvia, the Irtysh River right bank downstream Pavlodar at Grigoryevka village, 5238′ N 7644′ E, 24 iv 2016, N. Akimbekova leg. ; 1 ♀, Irtysh River right bank at Terenkol (formerly Kachiry) village, 5304′ N, 7606′ E, 24 iv 2014, I. Sivec, N. Akimbekova leg. ( PSU).
Remarks. The above males are in agreement with the detailed descriptions and figures of T. araneoides from the Danube River (Klápalek 1902, 1905; Bianchi 1905; Zhiltzova 2003) exhibiting diagnostic features of the species, the vesicle situated in the apical half of sternite IX, tergite X not incised apically ( Fig. 2 View FIGURE 2 d); the outer portion of the paraproct expanded basally and acute apically, curved at the tip ( Fig. 2 View FIGURE 2 c), the cerci produced to an inner tooth ( Fig. 2 View FIGURE 2 c–f), in front of which there is a tiny projection (the vestigial second segment of the cercus) ( Fig. 2 View FIGURE 2 c–d), a large upright epiproct ( Fig. 2 View FIGURE 2 c). The male habitus ( Fig. 1 View FIGURE 1 a, 2a) is identical to a photograph of a specimen in the Hungarian Natural History Museum, Budapest, collected at the beginning of the 20th century at Budapest ( Kovcs & Murányi 2008: fig. 1).
The female has no transverse vein in the subcostal field ( Fig. 3 View FIGURE 3 a) as found in T. araneoides and in contrast to T. nebulosa with a crossvein (Klápalek 1902, Bianchi 1905, Zhiltzova 2003). The above diagnostic features confirm our species identification. Even if there are some subtle differences from the presently extinct European populations, they cannot be compared without available fresh European specimens.
It should be noted that the series from Omsk includes eight males with exposed cerci not fully exposed and the epiproct still concealed inside a nearly flatly truncated tip of abdomen. At least three males had the epiproct small and rounded ( Fig. 2 View FIGURE 2 f). Presumingly, these specimens were collected soon after emergence.
Habitat and behavior. At Omsk, the Irtysh River has a strong current but not fast flowing, approximately 300–500 m wide, with sandy loam (a mixture of clay and sand) bottom and banks and rather turbid water. According to the limited observations by OK at Omsk, T. araneoides inhabits the main stem of the river, including at the city center, as well as large side channels. Adults of T. araneoides began emerging immediately after ice break in late April, caused by a sudden increase in discharge and followed by massive drifting of ice (Fig. 4). This sudden release was initiated several days before natural ice breakup by use of explosives upstream from the city to break up the ice to reduce flooding. In the city centre, the ice does not cover the river completely throughout the winter. Usually a large open water area along the right bank occurs because of inflow of warmer municipal waste water discharge. After ice break, or occasionally before at open water areas, larvae could be observed swimming at the water surface towards the bank by intensively wiggling their bodies, often in large numbers until early May. The following dates of the larval observations were: 13 IV 1975 (one individual) (ice break on 17 IV 1975); 22 (one) and 24 (several) IV 1977 (ice break on 18 IV 1977); 28–29 IV 1978 (one each day) (ice break on 27 IV 1978); 23 (one) and 29 (numerous) IV, 6 (extremely numerous) and 8 (few) V 1979. Upon reaching the bank, the larvae move to concrete structures for 10–20 minutes until finding shelter in cracks or crevices in the concrete or among driftwood or gravel, where adult eclosion takes place. Apparently, female larvae eclose only into adults in deeper, shaded-sheltered sites, whereas male larvae eclose among gravel or even in more exposed areas. Three larvae were observed after reaching the concreted river embankment on 29 IV and 6 V 1979 crawling 1–2 m above the water, then turning and approaching the water again and eventually eclosing close to proximity of the water’s edge.
The dates of observation of adults were as follows: 24 IV–5 V 1975; 26 IV 1977; 28–29 IV 1978; 26 IV–8 v 1979; 2–4 V 1995; 1–3 V 2003; 29 IV–2 v 2006; 1–4 V 2007. On 24 IV 1975, a male was found active on a concrete parapet when the air temperature was 0˚ C. In the first days of emergence, the males predominated, walking over the sandy river bank or on concrete embankments (e.g. Fig. 4) a dozen meters from the water edge, and congregating up to 30–40 individuals under driftwood or in cracks or crevices in the concrete embankments near the water’s edge. In these aggregations, a portion of males had the epiproct still unfolded and a few were teneral (e.g. on 2 V 1995, 3 V 2003). On two separate occasions (on 24 IV and 4 V 1979), captive males were observed to stop walking from time to time for a brief period to produce several quick drumming beats with the tip of abdomen upon substrate, as has been reported in this genus (Stewart et al. 1998). Mating was observed once on 4 V 1979. Males appeared uninterested in all females, but one with crumpled wings and yellowish liquid under it (just eclosed or injured?). Several males mounted her one by one for a short period of time, whereas one was observed mounted for a longer period of time.
At later dates of adult occurrence, females became more abundant. In sunny weather they could be observed gliding towards the river bank fluttering their wings over the water surface. Several females would be observed resting on the water surface for periods of time. Females would fold their wings when reaching the bank (observations of 1 V 2003 and 4 V 2007). Only once (3 V 2003) a female was observed flying over the water directly to the river bank, most likely returning after oviposition.
According to observations by NA, T. araneoides was abundant at the Irtysh River upstream of the city of Pavlodar on 19–30 VI 2014 –2016, but has not been collected within the city limits. Taeniopteryx araneoides also occurred downstream of the city but surrounding steep bluffs made observations difficult including attempts to estimate the relative abundance of this species. Females were found on grasses (mostly Poaceae ) growing immediately at the river edge. During the first days of adult emergence, many males would hide in loose sand of a small bluff, later wandering in search of females and periodically drum. Adults of T. araneoides were found mostly in the morning. No larvae were found at depths up to 0. 5 m. It is noteworthy that at Pavlodar, not a single individual was found along the left bank of the Irtysh River, possibly because the main flow of the river is close to the right bank, whereas at the left bank, the current is slow.
Discussion. The 4,248 km long Irtysh River flows from its source at the western slope of the Mongolian Altai Mountains at 4725' N, 9013'' in the Xinjiang Autonomous Region of China, traversing through the territory of Kazakhstan to Russia, passing the large Lake Zaisan and a series of three adjacent Bukhtarma, Ust’Kamenogorsk and Shulbinsk reservoirs, and enters the Ob River at 6105' N, 6850''. There are five large cities along the river, Oskemen (Ust-Kamenogorsk), Semey (Semipalatinsk) and Pavlodar in Kazakhstan and Omsk and Khanty- Mansiysk (at the mouth) in Russia. Taeniopteryx araneoides is not known from the Irtysh River further upstream of Pavlodar, e.g. at the city of Oskemen, where the river becomes more rapid, as well as downstream of Omsk. Devyatkov (2002) did not include T. araneoides in his checklist of stoneflies of the Upper Irtysh Basin. Additionally, occurrence of this species has not been confirmed from the large Yenisei River in Central Siberia, as suggested by a record by Zapekina-Dulkeit (1957, 1971a) from Krasnoyarsk, without specifying the name of the river, half a century earlier. Recent studies of stoneflies at the origin of the Yenisei River at the junction of the Biy Khem and Kaa Khem rivers in Tuva ( Zaika 2011) did not list this species.
Taeniopteryx araneoides is obviously a species of large rivers (a potamon species), as the Danube, Dniester, Elbe rivers in Europe, the Irtysh River in West Siberia and Kazakhstan and perhaps, the Yenisei River in Central Siberia. These rivers historically have been impacted by water pollution likely causing the extinction of of T. araneoide s in Central Europe during the 20th century. Omsk is a city with a population of 1.18 million people and includes a large industrial centre. Nevertheless, T. araneoides appears to be surviving well, even at the center of city. This species, however, has not been collected within the city limits of Pavlodar, a city of third the size, with a population of 335,000 people.
The geographical distribution of T. araneoides appears to be enigmatic, with a disjunction of 3,200 km between the Dniester and Irtysh rivers, and the seeming absence of this species from the Ob River at the city of Novosibirsk in West Siberia situated at the same latitude. The Ob River is similar to Irtysh River and is just 600 km east of Omsk. The reports of T. araneoides by Zapekina-Dulkeit (1957, 1971a, b) from Krasnoyarsk (further 650 km NEE of Novosibirsk), however, suggests that T. araneoides may occur at the next major river to the east, the Yenisei. The common Palearctic T. nebulosa is distributed from the Atlantic to Pacific coasts of Eurasia ( Zhiltzova 2003), but has not been recorded from the Irtysh River either at Pavlodar or Omsk, but is common at the Ob River and its ttributaries, the Inya River and Berd River. There is also a female specimen in the ISEA collected at the junction of the Inya and Ob rivers.
Another possible cause of the extinction of T. araneoides in Europe may be directly related to river regulation by dams and reservoirs preventing natural spring flooding that may serve as a trigger for adult emergence as supported by observations at Omsk. In this respect it is important to note that middle reach of the Irtysh River is still free flowing, with Pavlodar located 340 km and Omsk 730 km (air km) from the nearest upstream impoundment, the Shulbinsk Dam and reservoir. In contrast, the Ob River is dammed within the city limits of Novosibirsk, forming the large Obskoe Reservoir. It is also noteworthy that the reports of T. araneoides from Krasnoyarsk are from 1957–1971 ( Zapekina-Dulkeit 1957, 1971a), the Krasnoyarsk Hydropower Station (the 10th largest in the world) was constructed in 1956–1972, with T. araneoides collected just before the Yenisei River was dammed.
Taeniopteryx araneoides and T. nebulosa may be competing with and excluding each other in large rivers. Two ecogeographical scenarios may posited: 1) Two closely related and ecologically similar species most likely did not disperse over the Palaearctic simultaneously, whenever this dispersal occurred. It could be hypothesized that T. araneoides dispersed initially over its former range. Subsequently, T. nebulosa dispersed over the same extensive area and displaced T. araneoides from most large rivers. Taeniopteryx araneoides apparently maintained its stronghold in the Irtysh River (and probably Yenisei River) as well as in Central Europe, but later disappeared from the latter, at least in part, because of anthropogenic impacts. The fact that T. nebulosa still occurs in Europe, whereas T. araneoides became extinct, indirectly supports the assumption that T. nebulosa is more ecologically adaptive or tolerate, inhabiting a wide range of streams, from slow large rivers to smaller fast flowing rivulets and brooks ( Zhiltzova 2003). Such smaller streams may serve as refugia for T. nebulosa as water quality deteriorated in the larger streams and rivers due to anthropogenic perturbations. It is not surprising that T. araneoides , confined to large rivers, has persisted only in relatively pristine and unimpacted large lotic systems of Siberia and Kazakhstan, but disappeared from Europe. This interpretation assumes that T. araneoides is a relict species in currently known localities. 2) Both species could have dispersed over the Palaearctic at the same time or not, but T. araneoides is a species of large rivers and was more successful in these habitats than T. nebulosa . Taeniopteryx araneoides however was intolerant to river regulation and has been extirpated from these large rivers and far downstream from these impoundments, allowing T. nebulosa to occupy this open niche.
To test these hypotheses, more data of occurrence for both species in Siberia is required. One of the ways to test the above hypotheses would be reintroducing T. araneoides in the Danube River, now much less polluted than a century ago, using the Irtysh River population as a possible source. Hopefully, the Danube countries and European Union would be interested in such a restoration project attempting to recover their lost biodiversity. This attempt could reveal if ecological limiting factors for the species still occur in the Danube.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |