Ulyxes laertes (Domrow, 1972) Shaw, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3878.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:1A041046-5265-4E14-97F1-757A071EAF45 |
|
DOI |
https://doi.org/10.5281/zenodo.5142819 |
|
persistent identifier |
https://treatment.plazi.org/id/03F3EC13-FFE7-FF9E-FF0F-0A12BBF9FDDF |
|
treatment provided by |
Felipe |
|
scientific name |
Ulyxes laertes (Domrow, 1972) |
| status |
comb. nov. |
Ulyxes laertes (Domrow, 1972) new combination
( Figs 28−37 View FIGURES 28–29 View FIGURES 30–33 View FIGURES 34–37 , 57 View FIGURES 52–57 )
Haemolaelaps laertes Domrow, 1972a: 109 .
Haemolaelaps laertes . — Domrow 1988: 831.
Androlaelaps laertes . — Halliday, 1998: 123.
Specimens examined. 1 paratype female ( QM S58740 View Materials ), Canungra , Queensland, 5 Mar 1971, R. D(omrow) & R. W. C. coll. ex Rattus fuscipes View in CoL Bush Rat, 974 ; 1 female ( QM S58741 View Materials ), Canungra , Queensland, 15 Oct 1971, R . Domrow and R . W. C. coll., ex Rattus fuscipes View in CoL ; 1 female, Mt Nebo , Queensland, 28 Jan 1987, Steve Wilson, coll. ex Rattus fuscipes View in CoL ; 3 females, 2 males, Mt Clunie , New South Wales, 15 Jul 2001, J. Standing coll., ex small mammal nest in hollow log in woodpile, nest 409 ; 2 females, 1 male Tenison Woods Mt , 50km N of radiomast track 27º18’S 152º 45’E, 2–3 July 2003, F. Beaulieu coll., ex Asplenium australasicum View in CoL suspended litter in rainforest, 23 metres above ground ; 1 female, same data, but 11 metres above ground ; 1 female, same data, but moss and bark on decaying log. All in QM .
Females were described by Domrow (1972a).
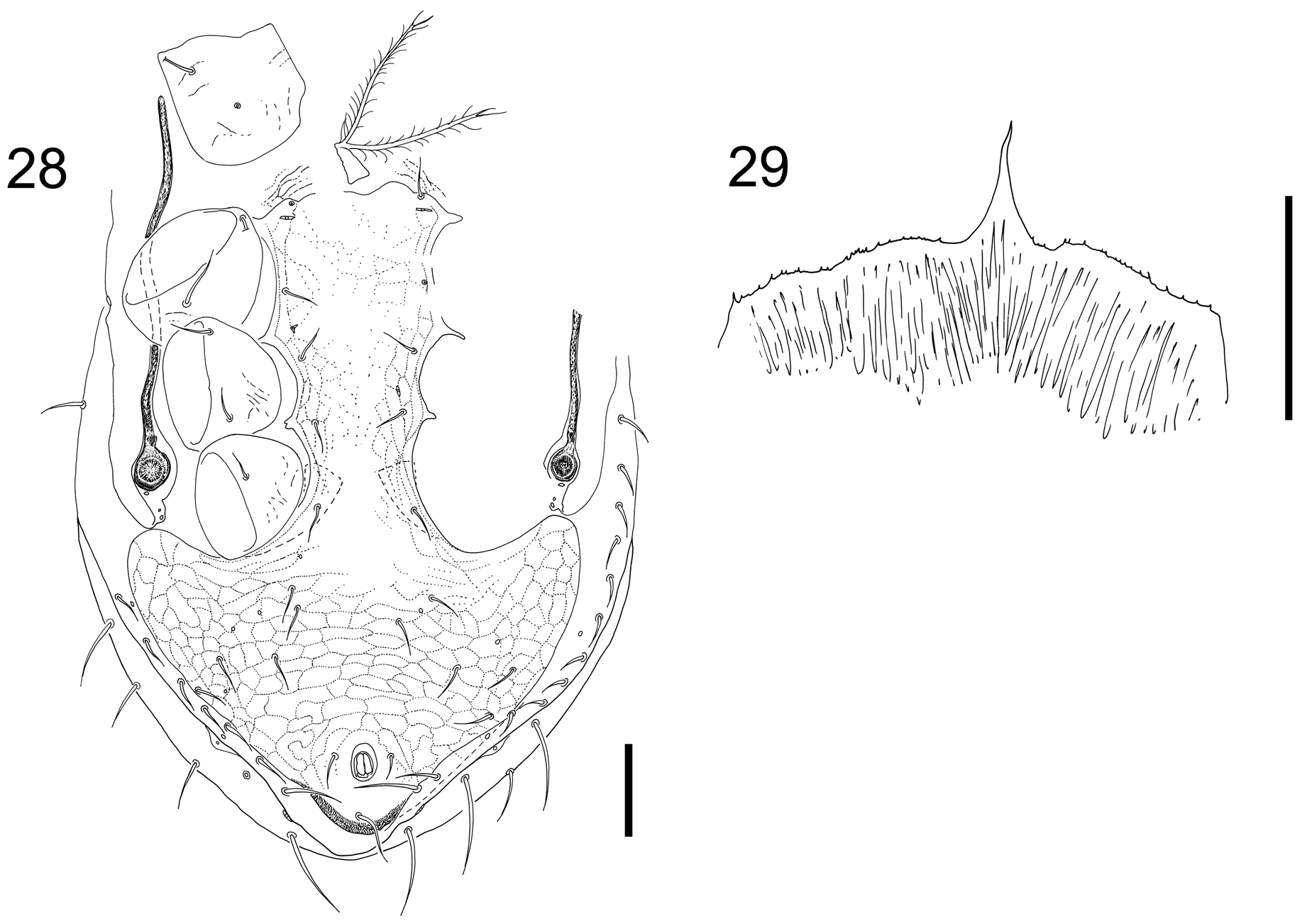
Description of males (n=3). Dorsum. ( Fig. 34 View FIGURES 34–37 ) Very large mites. Dorsal shield 1150–1190 long x 780–800 wide. 39 standard setae plus 3–4 unpaired Jx setae at level of J4. Podonotal j1 85–92, z1 ca. 42, other medial and discal setae short, e. g. j5 ca. 42, j6 ca. 47. J2 50, J5 50. Marginal setae heteromorphic in size, long and thick, increasing in length and becoming sabre-shaped posteriorly; s1 52, s2 60, r2 68, r3 90, r4 85, r5 87, S1 90–104, S2 95–98, S3 92–108, S4 90–120, S5 110–118. Dorsal shield covered in fine reticulations, fainter in mid-podonotal region.
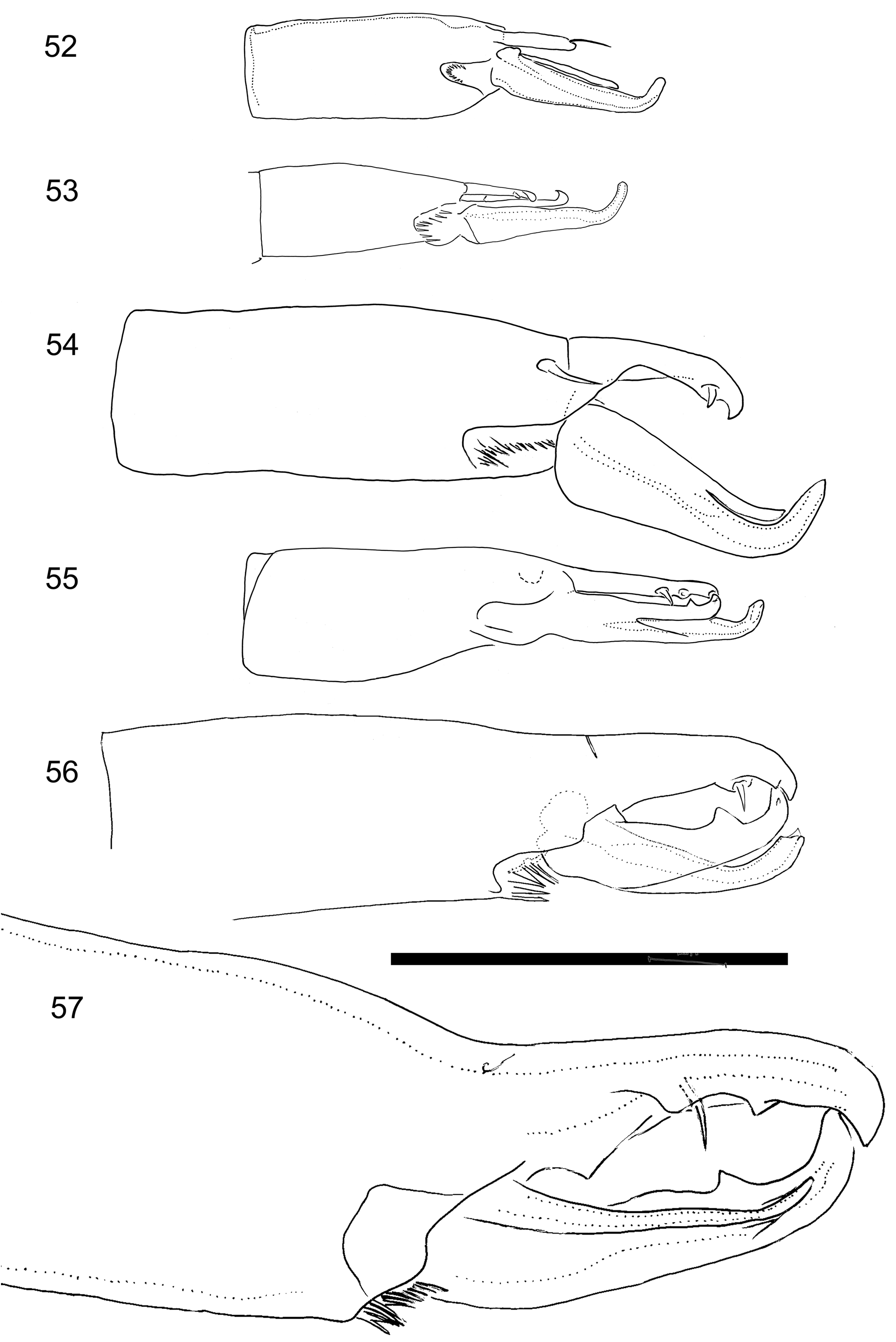
Gnathosoma . ( Fig. 32 View FIGURES 30–33 ) Palp genu al1 broad, 8µm maximum width, hyaline, lateral edges subparallel. Palp genu al2 narrow with long spatulate tip. Row Q1 bare, followed by usual six denticulate deutosternal rows (Q2–Q7) and bare Q8. Movable digit 188–205 long bearing single tooth. Spermatodactyl relatively small, shorter than movable digit with outline of movable digit completely encompassing that of spermatodactyl ( Figs 30 View FIGURES 30–33 , 35 View FIGURES 34–37 ). Most of spermatodactyl, 80% of length, fused with movable digit. Fixed digit without divided tip, with three strong teeth with pilus dentilis adjacent to second tooth. Cheliceral seta dorsal. Cheliceral segment II 415–430, segment I ca. 152. Epistome denticulate, narrows abruptly, leaving a narrow-based gradually-tapering medial point ( Figs 29 View FIGURES 28–29 , 36 View FIGURES 34–37 ).
Venter ( Fig. 28 View FIGURES 28–29 ). Tritosternal base 50–77 long to suture. Lacinae separate ca. 12 above suture and are free for ca. 212. Genital opening broad, very narrow oval, 65 wide x 22 deep. Opening set anteriorly, posterior edge level with st1. St1 65–80, st2 52–60, st3 52–53. Holoventral plate bears five pairs setae in addition to st1–5 and circumanal setae. Para-anal setae 48–50; postanal seta 73–80. Cribral pores separated by 197–230. Cribrum a dense beard of fine spicules, not in discernible rows.
Legs. Ten setae on trochanter I and femora I–IV modified as apically bifid ( Fig. 37 View FIGURES 34–37 ); trochanter 1 d, femur I pd2, ad3, femur II ad1, ad3, pd2, femur III ad1, pd1 and femur IV ad1, ad2. Femur II ventral setae unmodified. No modified setae on tarsus II. Pretarsal opercula with 10–12 tines. No apical stalk on leg I. Leg segment lengths as in Table 4 View TABLE 4 .
Remarks. Specimens of U. laertes were kept in culture for three months. Breeding occurred but no second generation adults were produced. Adult stages readily predated on adult Stratiolaelaps sp. Other prey that were accepted were a blood-fed endemic macronyssid protonymph and an anopluran louse. A female was also observed to grasp nematodes. The predatory habits of U. laertes match its unusually strong and large chelicerae. However, all postlarval stages also readily orientated towards sources of free blood and fed avidly from these. Judging by its chelicerae it seems unlikely that it would feed on unbroken skin of its host. Its attraction to, and ingestion of blood, could be an adaptation for utilising other sources of blood available within nests such as engorged mites, fed flea larvae, and the faeces of adult fleas. Engorged lice might also be available in the host’s fur.
In culture U. laertes showed a strong cryptic tendency, invariably hiding under squares of Parafilm provided for them. This behaviour could be a thigmotactic response. The mites did not seem to tolerate conspecifics in close proximity and apparent intraspecific aggression was observed on many occasions when a U. laertes would venture underneath a square of Parafilm and move too close to an already resident mite. The resident would make a short advance towards the intruder, with the intruder then changing direction and allowing the resident to maintain its position underneath its Parafilm shelter.
Ulyxes laertes has an enlarged capitulum ( Figs 32, 33 View FIGURES 30–33 ). The palp femora are internally concave ( Domrow, 1972a), and the epistome narrows abruptly creating a strong medial point ( Figs 29 View FIGURES 28–29 , 36 View FIGURES 34–37 ). These features appear to be accommodations for its enormously-enlarged chelicerae. Three cusps are found on the fixed digit of both females and males ( Figs 30, 31 View FIGURES 30–33 ) and in this respect, U. laertes appears unusually, though superficially, sexually monomorphic. Note that these dentitions between the sexes are not considered homologous as demonstrated by the different relative position of the pilus dentilis. The movable digits of females are three to seven times longer than other members of Ulyxes spp in absolute terms, and even as a ratio of body size they are two to more than four times longer ( Table 3 View TABLE 3 ). The digits are much larger than in any morphologically similar mites too, even including Laelapsella humi Womersley , that similarly-sized inhabitant of ground nests, whose digits are still only half as long as those of U. laertes .
This fascinating species is rare in collections. Prior to this study there were just three females in Queensland collections despite it being larger than most other symbiotic Laelapidae and despite it ocurring on small mammals that are frequently trapped by zoologists. I suggest its rarity in collections is because most collections of commensal arthropods are from hosts, and this mite is probably strongly nidicolous, rarely occupying its host’s fur. Ulyxes laertes was collected from two Crow’s Nest ferns at Tenison Woods Mountain. These ferns were used as nests by small mammals, judging by the co-occurrence of most life stages, including males and nymphs, of Mesolaelaps sp. in one nest, and the macronyssid Trichonyssus praedo (Domrow) in the other.
| QM |
Queensland Museum |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Ulyxes laertes (Domrow, 1972)
| Shaw, Matthew D. 2014 |
Androlaelaps laertes
| Halliday, R. B. 1998: 123 |
Haemolaelaps laertes
| Domrow, R. 1988: 831 |
Haemolaelaps laertes
| Domrow, R. 1972: 109 |