Acanthaspidia Stebbing, 1898
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3692.1.12 |
|
publication LSID |
lsid:zoobank.org:pub:22608F94-E70F-4148-A1DF-13D48A6F913C |
|
DOI |
https://doi.org/10.5281/zenodo.5690597 |
|
persistent identifier |
https://treatment.plazi.org/id/03F387EB-FF9C-FFC8-F1FF-249DFB80FA99 |
|
treatment provided by |
Plazi |
|
scientific name |
Acanthaspidia Stebbing, 1898 |
| status |
|
Acanthaspidia Stebbing, 1898 View in CoL
Acanthoniscus G.O. Sars, 1879: 434 p.; Wolff, 1962: 35 p. Iolanthe Beddard, 1886: 104 p.
Exacanthaspidia Menzies & Schulz, 1968: 171 p. Paracanthaspidia Menzies & Schulz, 1968: 161 p.
Type species: Acanthaspidia typhlops (G.O. Sars, 1879) , by original designation Gender. Female
Species composition. See Table 1 View TABLE 1 .
Remarks. To date, 20 Acanthaspidia species have been described (see Table 1 View TABLE 1 , incl. the new species). We included Acanthaspidia rostratus (Menzies & Schultz, 1968) to the list, although Brandt (1991) considered this species to be a synonym of A. drygalskii . Based on the investigation of type material of A. drygalskii , there are, however, slight morphological differences between A. rostratus and A. drygalskii , for example, in the length and shape of the rostrum (trifid in A. drygalskii vs. rounded in A. rostratus ), the lateral margin of pleotelson (blunt spines and deeply serrated in A. drygalskii vs. more smooth lateral margin in A. rostratus ), the body setation (with numerous setae on all body margins in A. drygalskii vs. very few lateral setae in A. rostratus ), the shape of the pereonites (acute in A. drygalskii vs. rounded in A. rostratus ), as well as the number of articles of the antennula (9 in A. drygalskii vs. 17 in A. rostratus , see also Menzies & Schultz 1968; Brandt 1991).
There is further evidence from molecular studies, in which Raupach et al. (2009) analyzed phylogenetic relationships among different isopod species and showed that A. rostratus is clearly distinct from A. drygalskii . Furthermore, Raupach & Wägele (2006) found A. drygalskii to represent a species complex. Initial micromorphological investigations of the specimens examined by Raupach & Wägele (2006) revealed differences in, for example, the rostrum shape (trifid, broad and pointed, and rounded), as well as the length of the mid-dorsal spines (authors pers. observ.). Whilst the number of specimens per haplotype was not sufficient enough to draw any final conclusion about intraspecific variations, these morphological and genetic investigations indicate that A. rostratus may, indeed, represent a distinct species.
Brandt (1991) argued that morphological differences between A. drygalskii and A. rostratus may be a consequence of different moulting stages. In fact, the specimens investigated by Brandt (1991) and Menzies & Schultz (1968) differ slightly in body size (Brandt, 1991: female 11.5 mm; Menzies & Schultz (1968): female 9 mm). Further studies combining molecular and morphological methods, as well as study of type material of A.
rostratus are needed to unravel systematic relationships among Acanthaspidia species. For now, we report A. rostratus as a distinct species until further investigations.
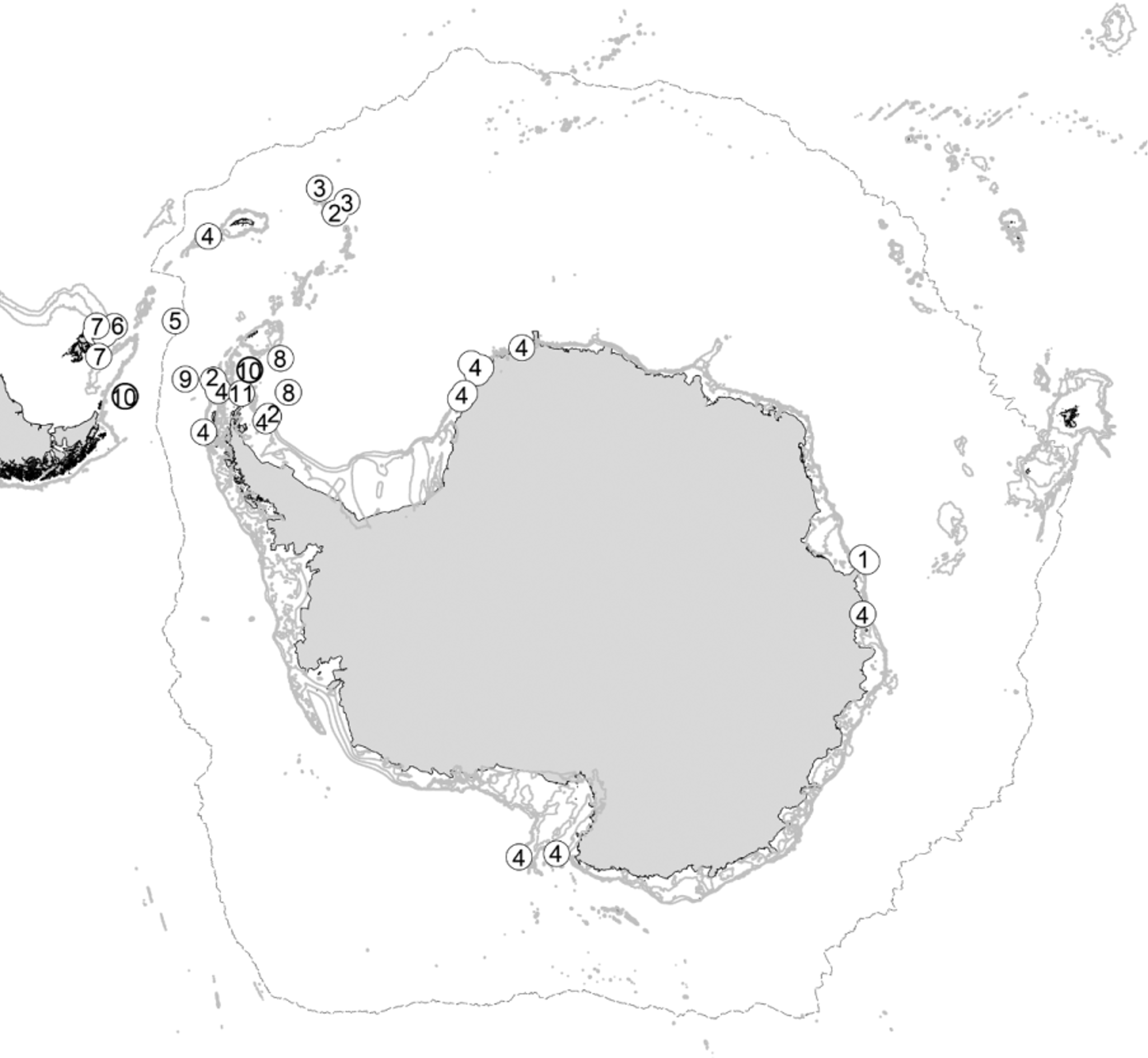
Distribution. Globally distributed, with most species occurring in the southern hemisphere ( Antarctic and Sub-Antarctic, South Africa, South Atlantic and South Pacific; see Table 1 View TABLE 1 ). Only four species have been described from the northern hemisphere ( A. typhlops , Acanthaspidia decorata Hansen, 1895 , Acanthaspidia laevis Chardy, 1975 and Acanthaspidia hanseni Birstein, 1963 ). In the Southern Ocean, most species have been recorded from the Atlantic sector (Weddell and Scotia seas), while only A. drygalskii sensu lato and A. acanthonotus (Beddard, 1886) seem to have a circum-Antarctic distribution ( Fig. 1 View FIGURE 1 ). Most Southern Ocean Acanthaspidia species are restricted to Antarctic waters, only Acanthaspidia sulcatocornia Menzies & Schultz, 1968 also occurs north of the Polar Front ( Fig.1 View FIGURE 1 ).
Generic diagnosis (modified from Brandt 1991; Just 2001). Head frontally with long rostrum, often longer than lateral margins of cephalothorax, without functional eyes; with prolonged, almost always pointed lateral projections of pereonites (length ≥ 0.2 pereonite width). Pleotelson broad, in many species often wider than pereonite 1, with robust lateral spines or at least deeply serrated. Pereopods subequal in shape. Antenna third peduncular article with strong exopod (squama), first flagellum article conjoint. Male and female pleopod 2 without prolonged setae distally.
Remarks. Acanthaspidia mainly differs from Ianthopsis by the lack of functional eyes and the prolonged lateral projections of the pereonites (length> 0.2 pereonite width). However, some species within Ianthopsis are blind too (such as Ianthopsis multispinosa Vanhöffen, 1914 and I. nodosa ) and some also possess prolonged lateral projections of the pereonites (length> 0.2 pereonite width, such as in I. multispinosa , Ianthopsis franklinae Brandt, 1994 and Ianthopsis vanhoeffeni Just, 2001 ). In contrast, in Acanthaspidia natalensis (Kensley, 1977) an elongation of the lateral pereonite projection cannot be observed. Brandt (1991) separated the two genera based the shape of the lateral margins of the pereonites (pointed in Acanthaspidia , rounded in Ianthopsis ); but, I. multispinosa and I. vanhoeffeni also have pointed lateral margins. Additionally, the length of the uropodal sympod has been used to distinguish both genera (Brandt 1991); in Acanthaspidia the sympod is longer than the rami, whereas in Ianthopsis sympod and rami are of equal length. In A. drygalskii sensu strictu, though, the uropodal sympod is shorter than the rami, while in Ianthopsis nasicornis Vanhöffen, 1914 , I. nodosa and Ianthopsis certus Kussakin & Vasina, 1982 the sympod exceeds rami length. Thus, the sympod-rami-length ratio does not seem to be a valuable character to separate Acanthaspidia and Ianthopsis . Furthermore, uropods have not always been illustrated or are broken off (such as in the new species) and therefore can often not be used as a distinguishing character. Brandt (1991) also considered the shape of the lateral margin of the pleotelson. The latter is smooth or only slightly serrated in Ianthopsis , while in Acanthaspidia the pleotelson bears long spines or is deeply serrated. However, in I. certus , Ianthopsis beddardi Kussakin & Vasina, 1982 and Ianthopsis studeri Kussakin & Vasina, 1982 the lateral margin of the pleotelson possesses robust spines.
Just (2001) discussed setation patterns of male and female pleopod 2 as a valuable character to distinguish both genera; in Ianthopsis the pleopods bear prolonged setae distally, while in Acanthaspidia lateral and distal setae equal in length or apical setae are lacking. Ianthopsis bovalli (Studer, 1884) , though, lacks prolonged distal setae on both male and female pleopod 2, whereas A. namibia Brandt, 2001 possesses prolonged apical setae on male pleopod 2. Hence, at this stage both Ianthopsis and Acanthaspidia do not seem to be defined by clear synapomorphies and thus are probably not monophyletic. This is further supported by molecular genetic analyses showing Ianthopsis and Acanthaspidia to be paraphyletic (Raupach et al. 2009). The material collected during several Antarctic cruises contains many species in need of further formal description and which are still not included in the diagnoses of the two genera (authors’ pers. observ.). Description of these new species and redescription of certain features (uropods in particular) and species (such as Acanthaspidia rostratus , A. drygalskii and A. decorata ) may help to define clusters within Ianthopsis and Acanthaspidia .
TABLE 1. Species composition and type locality of species within the genus Acanthaspidia Stebbing, 1898.
| Species Acanthaspidia | acanthonotus | (Beddard, 1886) | Type locality Depth (m) Kerguelen Islands 3062–3398 |
|---|---|---|---|
| Acanthaspidia | bifurcata | Menzies, 1962 | South-East Atlantic 2970 |
| Acanthaspidia | bifurcatoides | Kussakin & Vasina, 1982 | Scotia Sea, Antarctic 1729–1879 |
| Acanthaspidia | curtispinosa | Kussakin & Vasina, 1982 | Scotia Sea, Antarctic 6850–7219 |
| Acanthaspidia | decorata | (Hansen, 1895) | North Atlantic 4000 |
| Acanthaspidia | drygalskii | Vanhöffen, 1914 | East-Antarctic 350–385 |
| Acanthaspidia | hanseni | Birstein, 1963 | North-West Pacific 2940–3042 |
| Acanthaspidia | iolanthoidea | Kussakin & Vasina, 1982 | Scotia Sea 5600–6070 |
| Acanthaspidia | laevis | Chardy, 1975 | North Atlantic 1240–1200 |
| Acanthaspidia | longiramosa | Kussakin & Vasina, 1982 | Scotia Sea, Antarctic 720–2016 |
| Acanthaspidia | mucronata | (Menzies & Schultz, 1968) | near Falkland I. 567–864 |
| Acanthaspidia | namibia | Brandt, 2001 | Angola Basin, South-East Atlantic 5390 |
| Acanthaspidia | natalensis | (Kensley, 1977) | South Africa 1360 |
| Acanthaspidia | neonotus | (Menzies & George, 1972) | Peru-Chile Trench 5750 |
| Acanthaspidia | pleuronotus | (Menzies & Schultz, 1968) | Weddell Sea, Antarctic 3784–3788 |
| Acanthaspidia | porrecta | Menzies & Schultz, 1968 | Drake Passage 3722–3822 |
| Acanthaspidia | rostratus | (Menzies & Schultz, 1968) | Drake Passage 1455–1290 |
| Acanthaspidia | sulcatacornia | Menzies & Schultz, 1968 | West Scotia Basin, Antarctic 4008–4031 |
| Acanthaspidia | typhlops | (G.O. Sars, 1879) | Norwegian Sea 836–1416 |
| Acanthaspidia | matsi sp. nov. | this study | Powell Basin, Antarctic 1584 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |