Tyrannosauroidea
|
publication ID |
https://doi.org/ 10.1126/science.1193304 |
|
DOI |
https://doi.org/10.5281/zenodo.3809613 |
|
persistent identifier |
https://treatment.plazi.org/id/03F35F23-8F44-C006-E135-FED78BC9D855 |
|
treatment provided by |
Jeremy |
|
scientific name |
Tyrannosauroidea |
| status |
|
Here we assess the current state of tyrannosaur research, with a focus on the phylogenetic relationships and large-scale evolutionary patterns exhibited by the group, the biology of tyrannosaurs as living organisms, and information revealed from the newest discoveries.
Phylogenetic Relationships and Evolution of Tyrannosaurs
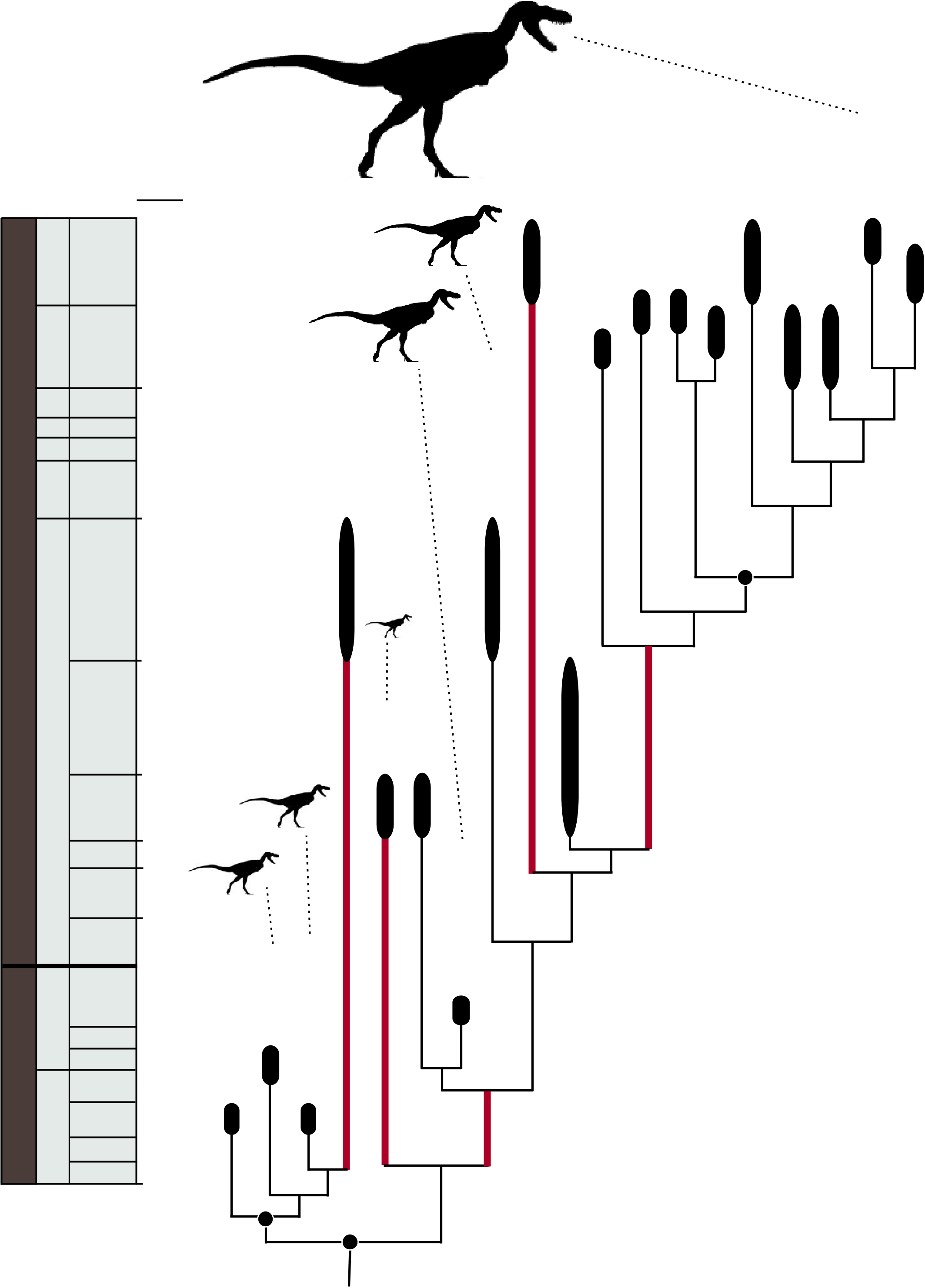
Tyrannosaurs, which formally comprise the clade Tyrannosauroidea , are a relatively derived group of theropod dinosaurs, more closely related to birds than to other large theropods such as allosauroids and spinosaurids (1, 17). Approximately 20 tyrannosauroid genera are currently known, 5 of which were described during the past year. To assess their interrelationships, we conducted a phylogenetic analysis, which includes all well-known genera scored for 307 morphological characters (18). The data set is based on personal observation of specimens and includes 123 novel characters (40% of total) based on recently discovered tyrannosaur taxa ( 4, 19 – 21).
Tyrannosaurs are a long-lived group that originated by the Middle Jurassic, ~165 million years ago ( 5) ( Fig. 2 View Fig. 2 ). The oldest and most basal tyrannosaurs comprise a speciose subclade, Proceratosauridae , which includes mostly small-bodied animals no larger than a human, many of which possessed elaborate cranial crests ( 22). Progressively more derived tyrannosaurs form a pectinate series on the line toward Tyrannosauridae , the subclade of multi-ton, deep-skulled behemoths from the terminal Cretaceous (Campanian-Maastrichtian), including Tyrannosaurus , Tarbosaurus , Albertosaurus , and close relatives (1). Taxa phylogenetically intermediate between proceratosaurids and tyrannosaurids include a range of genera from the Late Jurassic–early Late Cretaceous of Asia, North America, and Europe, most of which have been recently discovered ( 4, 14, 20, 21, 23, 24). These taxa run the gamut from small to medium size (~1.4 to 9.0 m in length), and few were likely apex predators in their ecosystems ( 21).
Until recently, the prevailing notion was that tyrannosaur body size gradually, and progressively, increased over time, in concert with the piecemeal accumulation of signature tyrannosaur skeletal features ( 14, 21). However, new discoveries have led to a reassessment. The Early Cretaceous proceratosaurid Sinotyrannus may have approached 10 m in body length, demonstrating that tyrannosaurs could attain a large size early in their history ( 25). More striking, the close tyrannosaurid outgroup Raptorex is only 2 to 3 m in length, suggesting that there was great size variability among close tyrannosaurid relatives and perhaps that the immediate ancestors of tyrannosaurids were small animals ( 4). Truly enormous size, however, is restricted to the latest Cretaceous tyrannosaurids, some of which grew to lengths of 13 m and masses of 5 to 8 tons ( 8). Therefore, for the first 80 million years of their history tyrannosaurs were mostly small- to mid-sized animals that lived in the shadow of other giant predators (e.g., allosauroids, megalosauroids), and only during the final 20 million years of the Mesozoic did they develop into some of the largest terrestrial carnivores to ever live ( 26). The dominance of tyrannosaurs as megapredators was purely a latest Cretaceous phenomenon.
Tyrannosaur Anatomy
The spate of new discoveries has prompted a renewed focus on tyrannosaur anatomy, including external, internal, and soft-tissue morphology ( Fig. 3 View Fig. 3 ). All tyrannosaurs are bipedal predators and possess several unique features, including a small premaxilla with D-shaped “incisor”-like teeth, fused nasals, extreme pneumaticity in the skull roof and lower jaws, a pronounced muscle attachment ridge on the ilium, and an elevated femoral head ( 6, 27, 28).
A number of derived specializations characterize the giant tyrannosaurids: a large and deep skull with powerful jaw muscles, robust teeth, reinforced sutures between skull bones, and tiny forelimbs ( 6, 29), features often considered adaptations for a hypercarnivore to function at large size ( 4). Basal tyrannosauroids, in contrast, have smaller skulls and longer arms and generally resemble sleek, bird-like theropods more than their enormous tyrannosaurid cousins ( 14, 22). New discoveries have shown, however, that the hallmark tyrannosaurid body plan (large and deep skull, robust teeth, etc.) does not uniquely or uniformly characterize the tyrannosaurid clade. Most of these features are now known to be present in Raptorex, the man-sized tyrannosaurid outgroup that lived 40 million years before tyrannosaurids originated ( 4). Furthermore, the gracile and long-snouted Alioramus, a tyrannosaurid that is about half the size of close relatives such as Tarbosaurus and Tyrannosaurus , lacks a deep and muscular skull and thick teeth ( 19). Thus, characteristic tyrannosaurid features did not evolve as a consequence of large body size, but likely originated in small animals, and not even all derived, Late Cretaceous tyrannosaurids are united by a characteristic morphotype ( 4, 19).
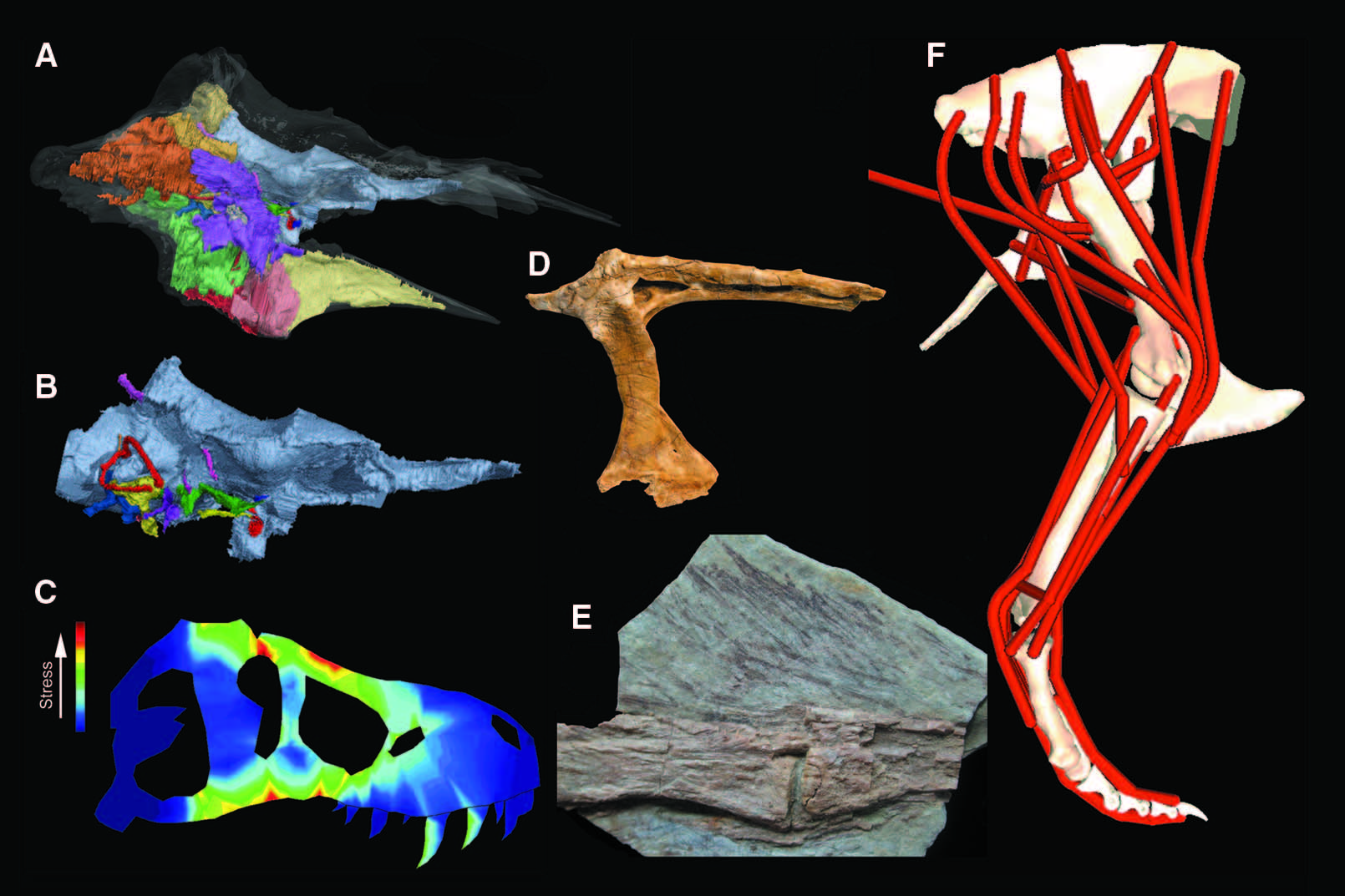
Much is also known about the internal anatomy of the tyrannosaur skull, thanks to the discovery of exceptionally preserved fossils and the application of digital techniques such as computerized tomography (CT) scanning ( 7, 30, 31). Tyrannosaurs possessed the required neuroanatomy to lead the active, predatory life-style expected of derived theropods ( 7, 30). Their encephalization quotient — an estimate of relative brain size — varies between 2.0 and 2.4, larger than in basal theropods but lower than that of birds and their closest relatives ( 19). Large olfactory lobes indicate a strong sense of smell ( 7, 31). Elongate cochlear and semicircular canals apparently support elevated sensitivity to low-frequency sound and highly coordinated head and eye movements ( 7).
Several tyrannosaurid specimens have been reported to preserve integumentary structures and other soft tissues, which rarely fossilize in dinosaurs. Although impressions of scaly skin have been described for large tyrannosaurids ( 32), simple filamentous integument, interpreted as homologous to feathers, is clearly preserved in a specimen of the basal tyrannosauroid Dilong ( 14). These branched filaments appear to have extensively covered the body, as they are observed near the skull and tail. A recent study suggests that much larger tyrannosauroids were covered with elongate, broader integumentary structures ( 33), which were likely used for display ( 34). Several easily degraded soft tissues, such as cells, blood vessels, and collagen,have been reported from a specimen of Tyrannosaurus ( 15, 35). Some of these findings have been met with skepticism ( 36), and they remain to be validated by other research groups. However, if correct, they promise to give radical new insight into the process of fossilization and may allow for molecular phylogenetic analysis of these extinct taxa ( 37).
Tyrannosaur Growth
Arguably we know more about tyrannosaur biology than that of any other dinosaurs ( Figs. 3 View Fig. 3 and 4). Much of this knowledge has been gained over the past 20 years, through the collection of skeletons of both adults and juveniles, bones of their prey with bite marks, coprolites (fossil feces), stomach contents, and pathological specimens ( 38).
Much attention has focused on how tyranno- saurs grew, especially on how giants such as T. rex achieved such massive size and how their skel- etons changed during the transition from embryo to multi-ton adult. Comparative growth curves for several species, which plot body mass (calculated from femur size) against age in years (calculated from counting growth lines in histological section) ( 8) ( Fig. 4B View Fig. 4 ), show that large tyrannosaurids reached somatic maturity around 20 years old, though most rarely lived for more than 25 years. T. rex evidently attained its large size via acceleration of growth rates relative to closely related species, not by extending its life span.Its maximum growth rate may have exceeded 767 kg per year, equiv- alent to adding a remarkable 2 kg per day ( 8). Tyrannosaur skeletons changed substantially as individuals matured. Although less is known about the growth of small, basal tyrannosaurs, tyrannosaurids and their closest large-bodied relatives are united by a conservative pattern of growth in which the skulls of juveniles were en- tirely reshaped during ontogeny ( 9, 20, 39). This sequence has been reconstructed by cladistic analysis, based on the principle that ontogeny, like phylogeny, involves a hierarchically nested series of character changes ( 19, 39). During the growth of an individual species, the skull and jaws deepened, pneumatic bones inflated, orna- mented structures enlarged and coarsened, sutural surfaces deepened and became more rugose, and the teeth became larger and thicker ( 9, 40) ( Fig. 1 View Fig. 1 ). Changes have also been documented in the post- cranial skeleton.Most notably, the forearm shortened and the long shin and foot of juveniles became shorter and stockier in adults ( 40). The differences between juvenile and adult tyrannosaurids are so great that different growth stages have often been mistaken for different species ( 3, 9). Tyrannosaur Behavior
A variety of studies have used biomechanical mod- eling, which incorporates mathematics, physics, and computer programming ( 41), to infer tyran- nosaur behavior. Tyrannosaurs, especially the large, derived forms, have often been used as exemplars to demonstrate the utility of such computer models.
Most studies have suggested that although large tyrannosaurids might have been able to run at slow to moderate speeds at best (top speeds between 5 and 11 ms −1), they could not run near- ly as fast as large athletic animals today, such as racehorses (~20 ms −1) ( 12, 41, 42). Consensus also is building that even though large tyranno- saurids were not restricted to a pillar-like columnar limb posture to maximize mechanical advantage, they were still far from having very crouched, more birdlike postures ( 12, 41, 42). Aspects of tyrannosaurid anatomy, such as the long legs and large pelvic limb muscles, which intuitively seem to indicate fast running capacity, were inherited from small, presumably fast-running ancestors. Modeling studies have incorporated these features and shown that they did not make large tyranno- saurids extremely fast. However, it is worth noting that these studies rely on estimates of muscle size and attachment points, a somewhat conjecturaexercise, albeit constrained by the anatomy of extant relatives ( 12, 16), that plagues all such functional analyses.
Both trace fossils (bite marks, coprolites) and quantitative techniques have helped to reveal what tyrannosaurs ate and how they fed. Tyrannosaurid bite marks have been found on the bones of a wide diversity of species, including various other tyrannosaurs, demonstrating that they were ecological generalists ( 43). Bite mark patterns show that tyrannosaurids characteristically bit deeply into carcasses, often through bones, and then pulled back, creating long cuts [puncture-pull feeding sensu ( 44)]. Some T. rex bite marks ( 44) and coprolites with bone chunks ( 45) indicate that bone was fractured, ingested, and used for sustenance, a mammal-like attribute not seen in extant reptiles. The bite forces needed to crunch through bone would have been enormous. Biomechanical experiments have replicated the size and depth of fossilized bite marks and suggest that T. rex generated bite forces of at least 13,400 N. Maximal bite forces were probably greater ( 46).
Such large bite forces would have exerted tremendous stress on the skull. Tyrannosaurid skull shape and its relation to bite-induced stress have been extensively studied by finite element analysis. The results indicate that large tyrannosaurids had skulls optimized to endure strong bites, as various sutures absorbed stress and the fused nasals strengthened the snout ( 11, 47, 48). Similar biomechanical techniques have also been used to examine the role of the tyrannosaur neck in feeding, showing that it was important for generating pulling forces on food items and in inertial feeding ( 49), and the function of the unusual “pinched metatarsus” of the foot in turning, indicating that it was structured to resist shearing and twisting forces ( 50).
Little is known about the ecological community structure for most extinct animals, but large sample sizes permit some understanding of tyrannosaur ecology. Late Cretaceous tyrannosaurids were the first dinosaurs for which population dynamics—the balance between deaths and births that create a population’ s age structure— could be assessed ( 10) ( Fig. 4C View Fig. 4 ). Like large birds and mammals, but unlike living reptiles, tyrannosaurids probably experienced extremely high neonate mortality, followed by few deaths after 2 years of age (presumably a release from predation), and then increased mortality at mid-life (probably from the rigors of reproduction), so that few individuals had a long reproductive life span. Furthermore, a number of fossil sites have preserved multiple individuals, suggesting that tyrannosaurs were at least occasionally gregarious ( 51). Bite marks indicate that individuals of the same species bit each other in the face during encounters ( 52), and many older individuals with gout, bacterial legions, and bone fractures have been reported, showing that disease and injury were common ( 53).
Multiple lines of evidence indicate that tyrannosaur ecological habits changed during ontogeny. In Late Cretaceous tyrannosaurids, the difference in form between the lightly built, fleet juveniles and the larger, bulkier adults suggests that foraging behavior and targeted prey size changed as tyrannosaurs grew. The deep and muscular adult skull, with reinforced sutures and robust teeth, is well suited for sustaining high bite forces, whereas juveniles had none of these features ( 9, 39). Furthermore, the longer and more gracile hind limbs of juveniles indicate that they were relatively faster than adults ( 40), which has been corroborated by biomechanical analysis ( 12). These differences could have promoted major size-related shifts in ecology and behavior. It is plausible that adults preferentially attack- ed larger, but less mobile, prey than their younger counterparts. Such an ontogenetic shift is not seen in many familiar predators today (e.g., lions), but is present in extant crocodylians ( 54). As most basal tyrannosauroids are similar in skull and body proportions to juvenile Late Cretaceous tyrannosaurids, it is likely that they behaved and fed in a similar manner. However, detailed biomechanical analyses have yet to be carried out for most nontyrannosaurid tyrannosauroids.
Whether T. rex and other large tyrannosaurs were scavengers or predators has generated much speculation and dispute. Bite marks from mass death assemblages of herbivorous dinosaurs show that tyrannosaurs scavenged on occasion ( 38). However, multiple reports of healed tyrannosaur bite marks on prey bones ( 55, 56) and tyrannosaur stomach contents containing remains of young dinosaurs ( 57) indicate that tyrannosaurs were capable of active predation. Like most carnivores, tyrannosaurs probably both scavenged and hunted.
One of the largest voids in our understanding of dinosaur biology is the sex of individual specimens. It has been suggested that female tyrannosaurs required a larger pelvic outlet for the passage of eggs, reflected by a greater span between the ischial bones and a smaller or more posteriorly located first tail chevron,but these indices find little neontological support in living archosaurs ( 58, 59). More recently, medullary bone, a calcium phosphate deposit for the use of shelling in eggs, was reported in one T. rex specimen ( 60). This provides a surefire identification of sex in dino- saurs, and holds much promise for future studies of dinosaur sex and ecology.
Tyrannosaur Biogeography
Until recently, all tyrannosaur fossils were limited to Asia and North America, but the discovery and recognition of basal tyrannosauroids over the last decade reveals a more cosmopolitan distribution during their early evolution ( 5, 22 – 24, 61). Members of the Middle – Late Jurassic proceratosaurid radiation are known from Europe and Asia ( 5), whereas the Late Jurassic genus Stokesosaurus is known from both Europe and North America ( 24). However, all well-known tyrannosaurs more derived than Eotyrannus and Stokesosaurus exhibit a purely Asian or North American distribution. Faunal interchange between these continents is characteristic of most Campanian-Maastrichtian dinosaur clades and reflects an increasing Laurasian- Gondwanan provincialism during the final stages of the Age of Dinosaurs ( 62). Tyrannosaurs, because of their rich fossil record and well-studied phylogenetic relationships, are one of the primary sources of evidence for this long-established biogeographic hypothesis.
Emerging evidence, however, indicates that tyrannosaurs were likely present on the southern continents during their early evolutionary history. An isolated pubis from the Early Cretaceous of Australia was recently identified as belonging to a derived tyrannosaur ( 13). As contemporary Earlymid Cretaceous dinosaurs mostly belong to globally distributed clades ( 26), the absence of Gondwanan tyrannosaurs during this time had been a puzzling anomaly. Even with this discovery, if it is from a tyrannosaur, tyrannosaurs are absent in the well-sampled mid-Late Cretaceous units of South America, Africa, and Madagascar ( 63). It is possible that tyrannosaurs were rare on the southern continents during the Early-mid Cretaceous, and it is likely that Gondwanan forms did not persist into the latest Cretaceous, at least as common and ecologically dominant carnivores.
Most tyrannosaurs are known from mesic (moderate moisture) or seasonally mesic paleoenvironments, and their fossils are notably absent from xeric (dry) facies, even those that interfinger with tyrannosaur-bearing mesic sediments within the same sedimentary rock basins in Asia ( 64). This likely indicates that tyrannosaurs preferred wetter habitats, although it may still reflect a sampling bias. Wherever they were present during the Late Cretaceous in North America and Asia, tyrannosaurs were the sole apex predators in their environments. Multiple large tyrannosaurids co-occurred during some intervals in North America and Asia ( 19, 27), but the Maastrichtian of western North America was solely dominated by T. rex ( 39). In contrast, most nontyrannosaurid tyrannosauroids are found alongside larger non- tyrannosaur predators, demonstrating that tyrannosaurs did not exclusively dominate the apex predator niche, regardless of where they lived, until the final 20 million years of the Cretaceous.
Conclusion
Tyrannosaurus rex and its close relatives are the most intensely studied dinosaurs. Derived tyrannosaurs such as Albertosaurus , Tarbosaurus , and Tyrannosaurus are known from more fossils than are most other dinosaurs, and these specimens span the spectrum from juvenile to adult. Many modern analytical approaches have been pioneered with the use of Tyrannosaurus and close kin, and the results of these studies are allowing for quantitative comparisons between the biology of extinct dinosaurs and living species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.