Miotoxaster collegnii ( Sismonda, 1844 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4656.1.5 |
|
publication LSID |
lsid:zoobank.org:pub:884B53B1-3EC1-4258-A4E6-1928F167632C |
|
persistent identifier |
https://treatment.plazi.org/id/03EF87BA-542D-3E3B-5595-F8F6FAA9DF7F |
|
treatment provided by |
Plazi |
|
scientific name |
Miotoxaster collegnii ( Sismonda, 1844 ) |
| status |
|
Miotoxaster collegnii ( Sismonda, 1844)
( Figs 6 View FIGURE 6 , 7 View FIGURE 7 , 8 View FIGURE 8 )
1844 Toxaster collegnii Sismonda , p. 361, pl. 1, figs 9–11.
1848 Toxaster micrasteriformis Gras , p. 60, pl. 4, figs 5, 6.
1853 Echinospatagus Collegnii d’Orbigny , p. 169, pl. 846.
1858 Enallaster Fittoni Desor , p. 357, pl. 40, figs 5–7.
1873 Echinospatagus Collegnii (Sismonda) —de Loriol, p. 350, pl. 30, figs 1–5.
1878 Enallaster Fittoni Forbes, Wright , p. 288, pl. 65, figs 1–2.
non 1878 Enallaster collegnii Sismonda, Wright , p. 283, pl. 64, fig. 4 [= Miotoxaster sp.].
1960 Toxaster collegnii Devriès , p. 30.
2002 Toxaster collegnii Sismonda, Villalba Currás , p. 112, pl. 2, figs 12–15.
2002 Toxaster gibbus Agassiz, Villalba Currás , p. 115, pl. 3, figs 1–5.
2008 Miotoxaster collegnii (Sismonda) , Smith & Wright, p. 578, pl. 185, figs 1–3, pl. 186, figs 1–3, pl. 187, figs 1–2, text-figs 244–247.
? 2011 Toxaster renevieri Wright , Taherpour-Khalil-Abad et al., p. 87, pl. 1, figs 1a–d, pl. 3, figs 1, 5.
? 2011 Toxaster granosus d`Orbigny, Taherpour-Khalil-Abad et al., p. 87, pl. 1, fig. 3a–d, pl. 3, fig. 3.
? 2011 Toxaster collegnii Sismonda , Taherpour-Khalil-Abad et al., p. 87, pl. 2, fig. 1a–c.
? 2012 Pliotoxaster collegnii (Sismonda) , Forner et al., p. 31, fig. 2a–e.
Material. The following 14 specimens from the vicinity of the village Gelian, NE Iran, (Sarcheshmeh Formation, early Aptian) were studied: GSINET 97FE234– GSINET 97FE247, from which the specimens GSINET 97FE234, GSINET 97FE237– GSINET 97FE238, GSINET 97FE240, GSINET 97FE243, and GSINET 97FE245–GSINET- 97FE247 were included in the biometric analysis.
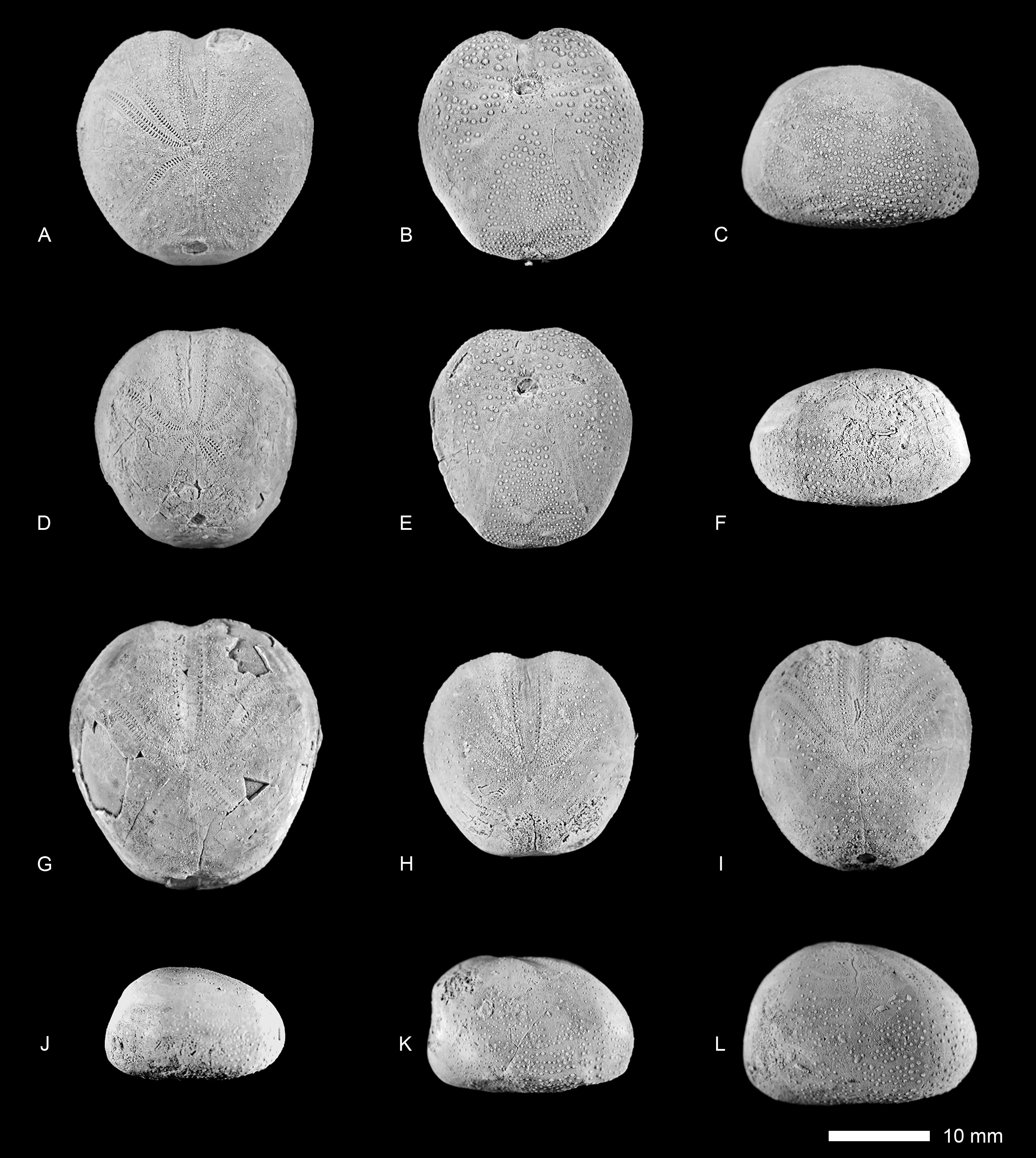
Diagnosis. A small-sized and relatively thin-shelled Miotoxaster species with adapically enlarged partitioned isopores in the anterior (unpaired) ambulacrum ( AIII). The aboral paired ambulacra are only very slightly sunken and form petals. The apical disc is subcentrally positioned. The posterior paired petals are short in comparison to the anterior paired petals. The posterior face is obliquely truncated; it slopes adorally. Scattered granules are present in the perradius of the paired petals.
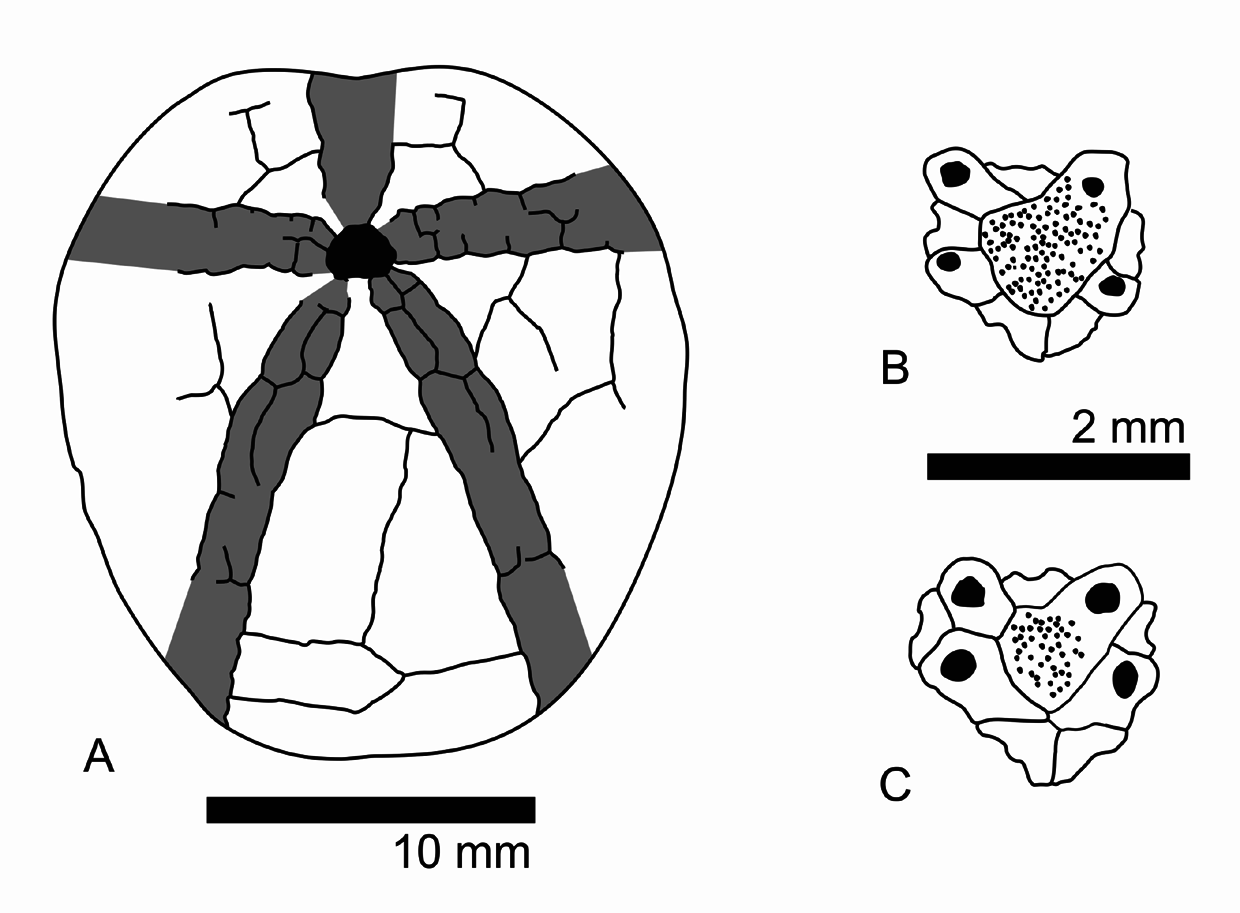
Description. Small species with a relatively thin test, with a maximum length of 26.9 mm. The outline of the test varies from elongate to subcircular (width is 91–101% of test length, mean = 0.95, n = 9). An anterior notch is faintly to moderately developed. The posterior face is obliquely truncated, with an anal angle of 66–82° (mean = 73°, n = 6). The tests are flattened to somewhat inflated (height is 61–71% of test length, mean = 67%, n = 9) with a flattened oral side. The tallest point of the test is located behind the apical disc, the position of which ranges from subcentrally to displaced to the posterior edge (50–59% of the test length, mean = 54%, n = 6). The apical disc has an ethmophract structure, with four gonopores. Genital plate 2 (the madreporite) is distinctly larger than the other genital plates. The posterior genital plates are either in contact with or separated from each other by genital plate 2. The posterior ocular plates meet behind the genital plates (see Figs 8 B, C View FIGURE 8 ).
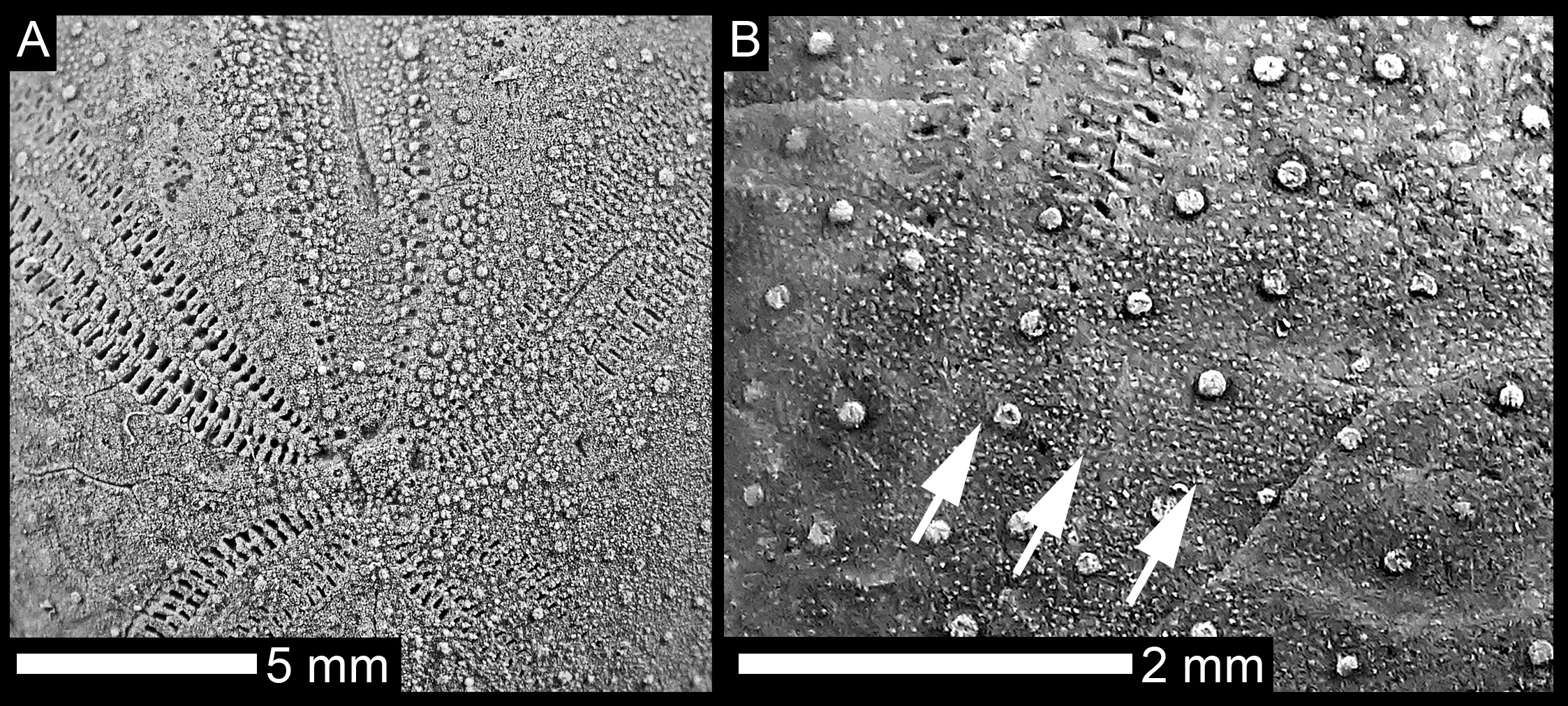
The paired ambulacra are petaloid, with only very faintly depressed petals. The anterior paired petals are relatively long, approximately 35–43% (mean = 38%, n = 6) of the test length and flexed in the proximity of the apex and distally, in the last third of their length. The pore pairs at the distal ends of the anterior petals are diverging, thus shaping an opened termination. The anterior column of the anterior petals is narrow with circular pores in the perradial row of the pore pairs and more elongated pores in the adradial side. The posterior columns of the anterior petals show well-elongated, conjugate pore pairs, with 53 pore pairs present in a single anterior petal at a test length of 18 mm and 68 at a test length of 24 mm, respectively. The perradius of the paired petals is smooth and wide, bearing few, scattered small granules.
The slightly curved posterior paired petals are relatively short, 16–21% (mean = 19%, n = 6) of the length of the anterior paired petals, and their pore pairs are conjugate showing elongated pores. At a test length of 18 mm, there are 29 pore pairs and 35 at a length of 24 mm respectively, in a single posterior petal.
The anterior ambulacrum is faintly to moderately sunken on the aboral side, with slightly oblique pore pairs. The pores of each pair are separated by a knob-like partition. The outer pore is somewhat elongated, while the inner pore is more circular.
The peristome is mostly wider than long and has a variable shape, from subcircular to subpentagonal in outline. It is marked by a smooth rim formed by the surrounding inter- and ambulacral plates. The peristome faces downwards or is slightly inclined towards the anterior region and its anterior border is located at a distance to the anterior edge of 18–26% (mean = 23%, n = 6) of the test length.
The plastron shows an amphisternous architecture. The triangular-shaped labral plate has a slightly concave contact to the larger sternal plate (5.a.2). Plate 5.b.2 has only a small contact with the labral plate. The asymmetrical sternal plates are separated by an oblique median suture. The labrum abuts with ambulacral plates I.a.2 and I.b.2.
The periplastronal ambulacra have a smooth, non-granular surface.
On the aboral surface, tubercles are scattered, set in a dense matrix of very minute granules. A protofasciole can be recognised between the anterior and posterior petals (characterised by horizontally aligned granules, sensu Néraudeau et al., 1998). Tubercles are more crowded, especially on the anterior-lateral, ventral-lateral, and plastronal plates of the oral side.
Remarks. Provisionally, we assign Toxaster collegnii to the genus Miotoxaster following Smith & Wright (2008), who distinguished Toxaster from Miotoxaster by the presence of subpetaloid pore pairs in the adapical part of the anterior ambulacrum, until a detailed revision regarding Toxaster , Miotoxaster , and the similar Pliotoxaster (the latter genus is distinguished from Miotoxaster in their more sunken petals), is available (compare Smith & Wright 2008). Epiaster d’Orbigny, 1853 can be delineated from Miotoxaster (as understood by Smith & Wright, 2008, in which we follow here) by the straight petals. The specific assignment of our material to M. collegnii is somewhat problematic as it was not possible to trace the type material of Sismonda (1844) and the description and illustration of Sismonda is brief and lacks detail (1844, p. 361, pl. 1, figs 9–11). However, the drawing of the type suggests that its general test shape and the size of its posterior petals are similar to those of the specimens described herein. Additionally, the described specimens have only faintly sunken paired petals and relatively short posterior paired petals, so match best with the material described by Smith & Wright (2008) from the British Aptian as Miotoxaster collegnii . However, our material deviates from previous interpretation of the test shape. The test shape in the Iranian material ranges from subcircular to more elongate and in lateral profile from flattened to a more domed upper surface, instead of being only subcircular in outline and having a domed apical-sided test. Additionally, the posterior genital plates are not separated by the madreporite of the apical disc in the British material ( Smith & Wright, 2008). This architecture occurs in herein described specimens together with the architecture in which the posterior genital plates are separated by the madreporite. This difference is likely not due to allometric changes, as all studied specimens are of a similar size. We believe that the differences between the Iranian and British specimens represent intraspecific variation as already mentioned for toxasterids ( François & David 2006) and can also be observed in other spatangoid echinoids (e.g., Schlüter 2016; Schlüter & Wiese 2017). Intraspecific variations should be considered in the much needed revision of toxasterid echinoids.
Taherpour-Khalil-Abad et al. (2011) reported also Toxaster renevieri and Toxaster granosus from the Tirgan Formation (late Barremian-early Aptian) from the Kopet-Dagh Basin. Toxaster renevieri was treated by Smith & Wright (2008) as a synonym to M. collegnii ; this view is followed here. Toxaster granosus deviates by its longer posterior paired petals and the more elongated pores in the aboral anterior ambulacrum from M. collegnii .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |