Trichogenes claviger, de Pinna & Helmer & Britski & Nunes, 2010
|
publication ID |
https://doi.org/10.1590/S1679-62252010000400002 |
|
persistent identifier |
https://treatment.plazi.org/id/03EE87F3-FFCB-BB40-FC51-1EC1FA5496FB |
|
treatment provided by |
Carolina |
|
scientific name |
Trichogenes claviger |
| status |
sp. nov. |
Trichogenes claviger View in CoL , new species
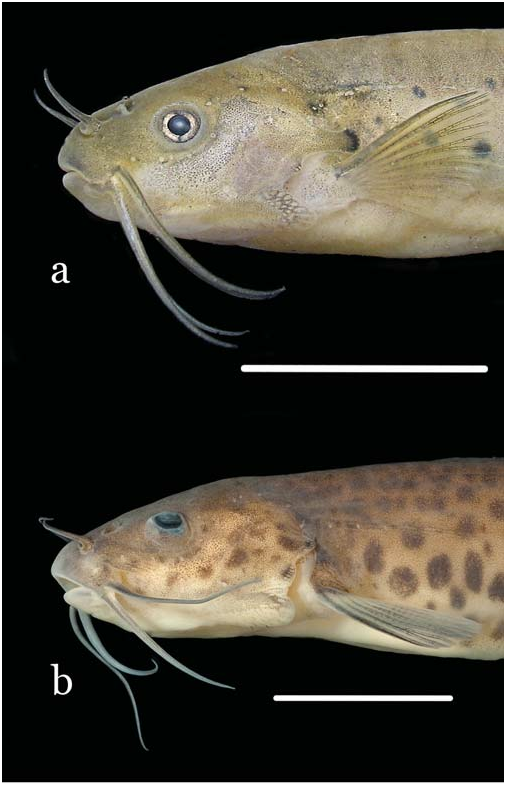
Figs. 1a View Fig , 2a View Fig , 3a View Fig , 4 View Fig , 5, 6a, 6b View Fig
Holotype. MBML 3289, 50.8 mm SL, Brazil, State of Espírito Santo, Municipality of Castelo, córrego Picada Comprida (tributary, in sequence, to ribeirão Braço Sul , rio Caxixe and rio Castelo ), rio Itapemirim drainage, 20°30’43”S 41°01’59”W, 15 Feb 2010, J. L. Helmer & L. R. Nunes. GoogleMaps
Paratypes. Total of 17 specimens, all collected with holotype. MBML 3290, 11 (1 c&s), 15.9-44.4 mm SL GoogleMaps ; MZUSP 105732 View Materials , 6 View Materials (2 c&s), 22.7-40.0 mm SL .
M. C. C. de Pinna, J. L. Helmer, H. A. Britski & L. R. Nunes 709
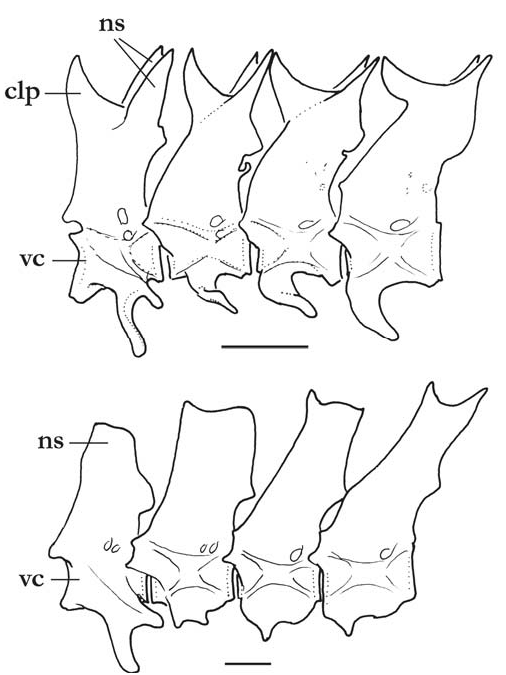
Diagnosis. Three autapomorphies unique among Trichomycteridae distinguish T. claviger from all other members of the family: the sexually dimorphic posterior process of the opercle, much elongated in males ( Fig. 2 View Fig ; vs. short in both males and females); the terminal mouth ( Fig. 1 View Fig ; vs. subterminal or inferior); and the presence of an anterodorsal claw-like process on the dorsal surface of the neural arch of each of the anterior four free vertebrae ( Fig. 3a View Fig ). Further distinguished from its only congener, T. longipinnis , by several additional characteristics (some of which may also be autapomorphic, pending more detailed analysis): the shape of the interopercle, with odontodes extending onto the posterodorsal margin of the interopercle on a bony expansion; ( Fig. 4 View Fig ; vs. odontodes mostly restricted to ventral and posterior margins of the bone); the posterior naris broader than long (vs. round); the presence of an entirely differentiated fleshy lobe laterally on the lower lip (vs. fleshy lobe mostly continuous with the lower lip); the lack of branched anal-fin rays in specimens of any size (vs. most anal-fin rays branched in specimens over 41 mm SL); the less deep caudal peduncle (9.3-11.5 vs. 10.3-12.6% SL); the deeper head (head depth 72.9-86.6 vs. 50.3-62.8% HL); the absence of an antorbital (vs. plate-like antorbital present dorsally to antorbital process of lateral ethmoid); the deep coronoid process of the lower jaw ( Fig. 5; vs. coronoid process approximately one-third less deep); the flattened bifurcated tooth cusps, with roundish margins ( Fig. 5; vs. bifurcated tooth cusps conical, pointed); the presence of 35 vertebrae (vs. 38 or 39); the presence of 6 branchiostegal rays (vs. 7); the absence of a pelvic splint (vs. pelvic splint present); the presence of 8 pleural ribs (vs. 10 or 11); the few sparse dark spots on body ( Fig. 1 View Fig ; vs. spots more numerous and more densely arranged); the well-defined thin dark line along base of anal fin, formed by a regular row of slanted elongate spots on the distal portion of each pterygiophore ( Fig. 1 View Fig ; vs. no such line); the lack of dark spots on the sides of head ( Fig. 1 View Fig ; vs. lateral surfaces of head with roundish spots); the dark spots on body not extending onto base of caudal fin ( Fig. 1 View Fig ; vs. spots covering bases of principal caudal-fin rays). The body shape of T. claviger , with the deepest part of the body at the middle of the abdomen, then continuously less deep posteriorly to the base of the caudal fin, and the dorsal and ventral profiles of the head forming broad symmetrical arcs with the body profile, result in a rather different general aspect when compared to T. longipinnis . In the latter, the deepest part of the body is at the origin of the anal fin, and the body depth is approximately even along its entire length, only slightly decreasing towards the caudal fin. Also, the dorsal and ventral profiles of the head and body are not symmetrical, with the former gently convex and the latter approximately straight.
710 A new species of Trichogenes from the rio Itapemirim
Description. Morphometric data given in Table 1. Body blunt, deeper than broad anteriorly and gradually more compressed posteriorly to caudal fin. Anterior part of body and head, from snout to dorsal-fin origin, in broad continuous convex arc. Dorsal profile of posterior region of body, from endpoint of dorsal fin to caudal-fin origin, mostly straight.Ventral profile broadly convex from snout to origin of pelvic fins, then straight along entire length of anal-fin base. Deepest part of body at middle of abdomen, then gradually less deep to base of caudal fin.
Head wide and deep, its depth approximately threequarters or more of HL. Mouth terminal, positioned at middepth of head, with jaws equally long or sometimes either upper or lower jaw slightly longer. Upper and lower lips narrow. Upper lip continuous laterally with maxillary barbel. Lower lip smaller and narrower than upper lip, subdivided into welldefined elongate fleshy lobe laterally, separating lip from base of rictal barbel. Dentary teeth 19-23, disposed in two rows. Outer row with 7 or 8 large, distally expanded, compressed and slightly tan-colored, bilobed teeth, with round cusp edges, gradually smaller laterally. Teeth on inner row smaller, less markedly expanded and less deeply bilobed than on outer one. Two rows mixing up laterally, with 2-4 lateral teeth conical. Premaxillary teeth similar to those on dentary, 20-22 in number and also disposed in two rows, with 6-8 teeth in outer row. Difference in tooth morphology between rows similar to that described for dentary. Two or three lateralmost premaxillary teeth of inner row conical. Many replacement tooth cusps alongside inner tooth row on both dentary and premaxilla.
Center of eye located slightly anterior to middle of HL, closer to lateral margin of head than to dorsal midline in dorsal view. Skin over eye thin and transparent, orbital margin free. Infraorbital latero-sensory canal complete, with five ossicles plus well-ossified lacrimal anteriorly. Five infraorbital pores, first one positioned posterodorsally to eye, second one posteroventrally to it, third and fourth ones along ventral margin of eye and fifth one directly anterior to that. Anterior naris surrounded by short anterolateral integument tube, continuous posterolaterally with nasal barbel. Posterior naris large and wide, broader than long in shape, located directly posterior to anterior one and partly occluded by two partly continuous flaps of skin. Three pairs of barbels, maxillary one maximally reaching base of first pectoral-fin ray. Rictal barbel inserted ventrally to maxillary
M. C. C. de Pinna, J. L. Helmer, H. A. Britski & L. R. Nunes 711
one, reaching posterior tip of interopercular patch of odontodes. Nasal barbel originating on posterolateral region of anterior naris, reaching anterior margin of eye. Opercular odontodes disposed in two separate groups ( Fig. 6 View Fig ). Dorsal group with 11-13 small odontodes in large specimens (5 or 6 in small specimens) at distal portion of well-defined opercular process with round expanded tip. Size of opercular process variable among specimens, and positive- allometrically related to body size and probably sexually dimorphic (see Discussion). Second small patch of opercular odontodes located anteroventrally to opercular process, bearing 2-5 tiny odontodes (one paratype entirely lacking second patch of odontodes on one side). Interopercle with well-developed patch of odontodes, visible in lateral and ventral aspect of head. Odontodes extending onto dorsal margin of bone, rendering its exposed, odontode-bearing, portion leaf-shaped. Interopercular odontodes 20-30, arranged in two series, main one extending alongside edge of interopercle, with large odontodes oriented obliquely to ventral margin of bone ( Fig. 4 View Fig ). Smaller series less orderly disposed, wih small odontodes interspersed among bases of large odontodes on main row. Odontodes extending onto posterodorsal portion of interopercle, forming well-defined roundish saw-like arrangement of erect small odontodes on prominent bone expansion ( Fig. 4 View Fig ). Interopercular odontodes less numerous in small individuals (13 in specimen 37.6 mm SL).
Pectoral fin large, with convex distal profile, its base immediately posterior to vertical through tip of interopercle, shorter than HL. Pectoral-fin rays i,9 (n = 9) or i,10 (n = 1), and some specimens with asymmetrical counts (n = 7, holotype). Pelvic fin with round distal profile, its origin slightly posterior to vertical through tip of pectoral fin. Pelvic-fin rays i,5 (n = 2) or i,6 (n = 11), and some specimens with those values differing on each side (n = 3; holotype), and single specimen with i,7 on one side and i,6 on the other. First pelvic-fin ray (unbranched) shorter than others. Pelvic splint absent. Dorsal fin smaller than anal and pectoral fins, its dorsal profile convex. Dorsal-fin origin closer to base of caudal fin than to tip of snout. Dorsal-fin rays i,7, plus single large procurrent ray anteriorly. Anal fin long, its distal profile straight or gently convex, with round posterior end. Last anal-fin ray adnate between 30-80% of its length. Origin of anal fin slightly anterior to middle of SL, its base longer than 50% of body length (excluding head). Anal-fin origin located slightly anterior to middle of SL. All anal-fin rays unbranched (except for single 6 th ray branched in one specimen), numbering 32 (n = 3), 33(n = 8), 34 (n = 5; holotype) or 35 (n = 1), plus 2 small procurrent rays. Caudal fin bilobed, with 7+8 principal rays. Upper lobe slightly longer than lower lobe in some specimens. Procurrent caudal-fin rays 9 or 10 dorsally and 6 or 7 ventrally.
Lateral line short, extending from posterior part of head almost to vertical through margin of pectoral fin, composed of an interrupted series of 4 or 5 short independent tubules, with small pore at each extremity. Each lateral-line segment with one delicate lateral-line ossicle, progressively less wellcalcified posteriorly. Vertebrae 35 (n = 16). First anal-fin pterygiophore inserted posterior to haemal spine of 10th free vertebra (n = 12). First dorsal-fin pterygiophore inserted posterior to neural spine of 16 th free vertebra (n = 11). Dorsalfin pterygiophores 8, anal-fin pterygiophores 32 or 33 (holotype). Pleural ribs 8, sometimes with small additional vestige posteriorly. Branchiostegal rays 6.
Coloration. Sides of body with scattered dark round or oval spots of different sizes, roughly small, medium and large ( Fig. 1a View Fig ). Largest and most conspicuous of such spots forming a series (9 to 13 in number) beginning posteriorly to opercular process and continuing obliquely ventrally to near the origin of the anal fin, then extending approximately in parallel to anal-fin base until end of caudal peduncle. Additional round markings scattered sparsely on sides of body, medium-sized ones concentrated near midline and smaller ones mostly on dorsal half of sides, not forming any specific pattern and mostly or entirely absent on dorsum. Dark spots of all sizes less numerous in small specimens, and totally absent in uniform dark grey smallest individual (15.9 mm SL). Some adult specimens showing only main row of large spots, lacking small and medium-sized ones. Dark spots not entering base of caudal fin. Entire body, except abdomen, with fine uniform background covering of small melanophores, abruptly denser dorsal to lateral midline and slightly denser along limits of 712 A new species of Trichogenes from the rio Itapemirim myosepta and on dorsal midline. Abdominal region white, except for narrow dark fields between bases of pelvic fins and a dense concentration anterior to origin of anal fin. Anterior portion of lateral line with amorphous dark cloud and small dark spot around one or two anterior pores. A well-defined dark line on body along base of anal fin, formed by series of partly coalescent, elongate and anteriorly slanted, dark markings on distal portion of each anal-fin pterygiophore. Dorsally and parallel to that line, long narrow band lacking dark chromatophores, forming white counterpart to it. Two vertical elongated fields, sometimes forming vertical bar, immediately anterior to base of caudal fin. Dorsal margin of caudal peduncle darker than surrounding areas. Head with uniform covering of fine chromatophores, densest on upper lip, nearly or totally lacking dark spots seen on body. Cheeks always lacking dark spots. Opercular process slightly darker than surrounding areas, its expanded tip with dark spot similar in color, shape and position to those on large series of spots on body. Small concentration of dark pigment on ventral patch of opercular odontodes. Integument amidst interopercular odontodes tinted with dark. Posterior naris white, surrounded by very dark rim. One or two temporal pores rimmed in dark. Nasal, maxillary and rictal barbels darkly-pigmented, with lighter cores. Nasal barbel darkest, except for its white basal narial tube. Maxillary barbel with dorsal surface darker than ventral. Rictal barbel lightest, with dark chromatophores at base and becoming progressively lighter distally. Ventral part of head with dark fields on region of lower jaw and margin of lower lip, with some scattered chromatophores also on branchial membranes. All fins with dark chromatophores alongside individual rays and segment limits, most pronouncedly along first pectoral-fin ray. Base of dorsal fin with irregular dark concentration. Base of caudal fin with welldefined semilunar dark field, apparently a continuation of the uniform background dark scattering on body.
The freshly-preserved specimens have a yellowish tint covering the dorsal part of head, including the infraorbital canal, the opercular process, the base of interopercular path of odontodes and most of the fins ( Fig. 2 View Fig ). The opercular region dorsally to the interopercular patch of odontodes is pinkish due to branchial blood seen by transparency.
Sexual dimorphism. The degree of development of the posterior opercular process in T. claviger is sexually dimorphic. Specimens 36.6 mm SL and larger fall into two well-defined classes with respect to the size of the process, large and small ( Fig. 6a,b View Fig ), with all specimens with the long process being males and all those with a short one, females.A total of 10 specimens were sexed (including 3 c&s ones), of which four were males and six females. The holotype was not directly sexed, but its long opercular process fits the morphology expected for males. In specimens 33.2 mm SL and smaller, no clear difference in proportional size of the opercular process was noticed. Both males and females have a dark spot at the tip of the opercular process and it is only the length of the latter which is sexually dimorphic. It is possible that the different lengths of the opercular process has a direct role in sexual signaling. Species of Trichogenes seem to be highly visually-oriented fish, and it has been shown experimentally that T. longipinnis will seek prey based on visual stimulus alone (Sazima, 2004). A black spot at the movable tip of a long opercular process is an obvious visual cue and, being sexually dimorphic, strongly suggests a sexual recognition function. A movable dark spot somewhere on the side of the body of the male is known to occur dimorphically also in other species of neotropical freshwater fish, such as in characids Corynopoma , Pseudocorynopoma and Pterobrycon . The spot-bearing anatomical structure varies among those taxa, and can be either modified opercular bones, the expanded tip of one or more pectoral-fin rays, or a hypertrophied scale, respectively (Bussing & Roberts,
M. C. C. de Pinna, J. L. Helmer, H. A. Britski & L. R. Nunes 713
1971), indicating that the strategy has evolved independently at least three times in glandulocaudines (N. Menezes, pers.comm.).
Distribution. Trichogenes claviger is known only from the type locality, an upland tributary of the headwaters of the rio Itapemirim drainage in southeastern Brazil ( Fig. 7 View Fig ).
Ecological notes. The type series was collected in a shallow sector (ca. 30 cm) of the córrego Picada Comprida ( Fig. 8), on a plateau at ca. 1150 m altitude. The water is darkly tea-stained and transparent, with slow current and negligible altitudinal gradient. The stream runs through an area of moderately impacted high-altitude rainforest mingled with sectors of exotic pine culture. The substrate is mostly exposed sand, with masses of accumulated leaf litter and other vegetable debris in many spots. Fish were concentrated on quiet shaded areas with litter, swimming in midwater, and were collected with hand seines. No other fish species seems to co-occur with T. claviger. Preliminary observation of gut contents revealed numerous disarticulated arthropod remains, indicating that the feeding habits of the species are broadly similar to those of T. longipinnis. Specimens of the species were also observed swimming in two other nearby spots in tributaries to the córrego Picada Comprida, but were not seen in four additional collection points in the same stream system and their distribution seems to be patchy. The type locality is situated between two State Parks: Forno Grande and PedraAzul, but lies outside of either, in private land. The exact geographical distribution of T. claviger is still undetermined, and more extensive fieldwork in the area will be necessary to obtain such information. In any event, it is certain that conservation measures will be needed to assure the survival of the species. The fact that T. claviger was discovered during a privately-commissioned environmental impact assessment demonstrates the importance of such initiatives for the knowledge of the freshwater fish fauna.
Etymology. Claviger means club-bearing in Latin, an allusion to the peculiar shape of the hypertrophied posterior process of the opercle in males of this species. An adjective.
Remarks. The geographical ranges of T. longipinnis and of T. claviger are separated by approximately 500 km in straight line, and by several intervening independent drainages along the Atlantic coast. The two species probably have had a long history of isolation, as judging by the large set of distinguishing characteristics in external morphology and internal anatomy, summarized in the Diagnosis above. Some of the diagnostic characters deserve comment. Vertebral number seems to be a consistent meristic difference between the two species of Trichogenes, with a well-defined gap in counts. Interestingly, very limited variation was detected in the material examined, with T. longipinnis having 38 (n = 9, holotype) or 39 (n = 5) vertebrae and all specimens of T. claviger with 35 (n = 18). The pattern of integumentary pigmentation in the two species of Trichogenes, although similar in general features, also consistently differ. Although much variation is seen among different populations of T. longipinnis (some of which is illustrated in Sazima, 2004), in no case does its pigmentation match the one in T. claviger. Part of the difference includes a dark line along the base of the anal fin of T. claviger. No such line is seen in T. longipinnis, although the distal portion of each of its pterygiophores appears as slightly darker than surrounding tissues due to the proximity of the bone to the surface of the skin. The resulting effect may look superficially similar to the dark line in T. claviger, but there is no dark pigment involved.
A single juvenile specimen of T. claviger has been collected so far (MBML 3290, 15.9 mm SL) and it displays some intriguing characteristics. The specimen has a very large eye, a compressed head, a very short snout, a prognathous mouth with the lower jaw produced and strongly oblique, and a markedly sloped anterior profile of the head ( Fig. 9a View Fig ). All those characteristics contrast with the situation in similarly-sized specimens of T. longipinnis , where the eye is comparatively small, the head is depressed, the snout is relatively long and the mouth is subterminal, with the lower jaw horizontal and included in the upper one, and the profile 714 A new species of Trichogenes from the rio Itapemirim of the head is gently sloped ( Fig. 9b View Fig ). Evidently, juveniles of T. longipinnis are more similar to their respective adults than young of T. claviger to theirs. Juveniles of Copionodontinae (cf. Campanario & de Pinna, 2000; pers. obs.) and at least some Trichomycterinae (cf. Lundberg et al., 2004) also do not differ markedly from their respective adults. The morphology of young T. claviger is thus deviant amidst its immediate phylogenetic neighbors, and is most likely an autapomorphic modification of the juvenile stage of the species. Of course, few juveniles of Trichomycteridae have been described and this hypothesis awaits confirmation. In any event, the young stage of T. claviger differs markedly from those of Siluriformes in general (cf. Nakatami et al., 2001; Lundberg et al., 2004; Leite et al., 2007). The quite distinct head and mouth morphologies also suggest different juvenile ecologies. Young and mid-sized specimens of T. longipinnis have been shown to be preferential daytime foragers and visually-oriented towards food items (Sazima, 2004). It seems likely that such traits will also be present in T. claviger , perhaps even more pronounced. It is also expected that their feeding behavior will be more strongly directed towards surface- and midwaterpicking. Sazima (2004) suggested that T. longipinnis , at least while juvenile and mid-sized, partly play the ecological role of a tetra (species of Astyanax and related small characins), i. e., diurnal, nektonic and insectivorous. This idea is supported by the apparent competitive exclusion of T. longipinnis and Astyanax spp. , the latter present throughout the general area of distribution of the former, but locally absent in its exclusive steep stream sectors. The physical stream characteristics have been proposed as a barrier preventing the establishment of Astyanax populations in T. longipinnis localities (Sazima, 2004). The more characin-like morphology (and putatively, habits) of T. claviger , especially as a juvenile, suggests a similar history of association with characin-free habitats. However, the environment of the new species is not steep water courses as that of its congener, but rather slow sectors of streams with little altitudinal gradient. It is not yet known which factors have kept species of Astyanax and other fish from entering the habitat of T. claviger .
M. C. C. de Pinna, J. L. Helmer, H. A. Britski & L. R. Nunes 715
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |