Vates phoenix, Rivera & Herculano & Lanna & Cavalcante & Teixeira, 2020
|
publication ID |
https://doi.org/ 10.5852/ejt.2020.598 |
|
publication LSID |
lsid:zoobank.org:pub:8BE6D600-5575-415B-8D90-6041B74408C0 |
|
DOI |
https://doi.org/10.5281/zenodo.3665161 |
|
persistent identifier |
https://treatment.plazi.org/id/EB7BB1D8-786F-401C-B408-2D41260C7797 |
|
taxon LSID |
lsid:zoobank.org:act:EB7BB1D8-786F-401C-B408-2D41260C7797 |
|
treatment provided by |
Plazi |
|
scientific name |
Vates phoenix |
| status |
sp. nov. |
Vates phoenix sp. nov.
urn:lsid:zoobank.org:act:EB7BB1D8-786F-401C-B408-2D41260C7797
Figs 2–5 View Fig View Fig View Fig View Fig , 6 View Fig A–H, 8–9; Table 1 View Table 1
Diagnosis
The new species can be easily recognized by the following combination of characters: i) cuticular projections above lateral ocelli strongly reduced (almost entirely missing in some specimens); ii) antennomeres of males asymmetrical (s-shaped); iii) hindwing of females with large, yellowish white and partially opaque area that spreads over most or part of the membrane; iv) anterodorsal lobe of hind tibiae at least 50% the length of tibial length (i.e., not narrowly restricted to its middle section).
Etymology
The specific epithet refers to the Phoenix, a mythical, immortal creature that is born again from its own ashes after being consumed by fire. The new species is a homage to the Museu Nacional of Rio de Janeiro, which was destroyed during a massive fire on September 2, 2018. The entire entomological collection, representing more than 5 million specimens, was destroyed, including all praying mantis specimens. Only a few specimens of Vates borrowed in the context of this study, including our new species, survived the event. Vates phoenix sp. nov. thus symbolically attempts to link the past and the future of the Museu Nacional, as it represents the rebirth of the Mantodea collection and our hopes for the revival of an even stronger institution in the not too distant future.
Material examined
Holotype
BRAZIL • ♂; Rio de Janeiro, Valença, Fazenda Recanto ; 22°07′15.3″ S, 43°51′01.2″ W; alt. 560 m; 15 Nov. 2015; Projeto Mantis leg.; white cloth light trap; MNRJ-ENT6-28441 . GoogleMaps
Allotype
BRAZIL • 1 ♀; Rio de Janeiro, Rio de Janeiro City, Jardim Botânico do Rio de Janeiro; 22°96′73.717″ S, 43°22′50.381″ W; alt. 7 m; 29 May 2018; M.L.F. Teixeira leg.; manual collection; MNRJ-ENT6-28442 .
Paratypes
BRAZIL – Rio de Janeiro • 1 ♂; same collection data as for holotype; MNRJ-ENT6-28445 GoogleMaps • 1 ♂; same collection data as for holotype; 31 Dec. 2016; white cloth light trap; William Moura leg.; MNRJ- ENT6-28443 GoogleMaps • 2 ♂♂; Reserva Ecológica de Guapiaçu, Cachoeiras de Macacu ; 22°27′10.309″ S, 42°46′13.011″ W; alt. 37 m; 18 Dec. 2017; Projeto Mantis leg.; white cloth light trap; MNRJ- ENT6-28446 , MNRJ-ENT6-28447 GoogleMaps • 1 ♂; Rio de Janeiro City, Corcovado ; 22°57′06″ S; 43°12′37″ W; alt. 600 m; Jan. 1936; D. Mendes leg.; MNRJ-ENT6-28448 GoogleMaps • 3 ♂♂; Angra dos Reis, Jussaral Train Station (note: now in ruins, the station closed down in 1996); 22°56′26″ S, 44°16′26″ W; alt. 351 m; Sep. 1934; D. Mendes leg.; MNRJENT6-28450, MNRJ-ENT6-28453 , MNRJ-ENT6-28454 GoogleMaps • 3 ♂♂; same collection data as for preceding; Sep. 1935; D. Mendes leg; MNRJ-ENT6-28449 , MNRJ-ENT6-28451 , MNRJ-ENT6-28452 GoogleMaps • 1 ♀; same collection data as for allotype; May 1935; MNRJ-ENT6-28455 . – São Paulo • 1 ♂; Angatuba ; 22°56′26″ S, 44°16′26″ W; alt. 7 m; Nov. 1917; A. Marques leg.; MNRJ- ENT6-28456 GoogleMaps .
Description
Male (holotype; MNRJ-ENT6-28441)
HABITUS. Live specimen (paratype) in Fig. 2A View Fig ; pinned specimen (holotype) in Fig. 2C. View Fig
MEASUREMENTS. See Table 1 View Table 1 (specimen FR01).
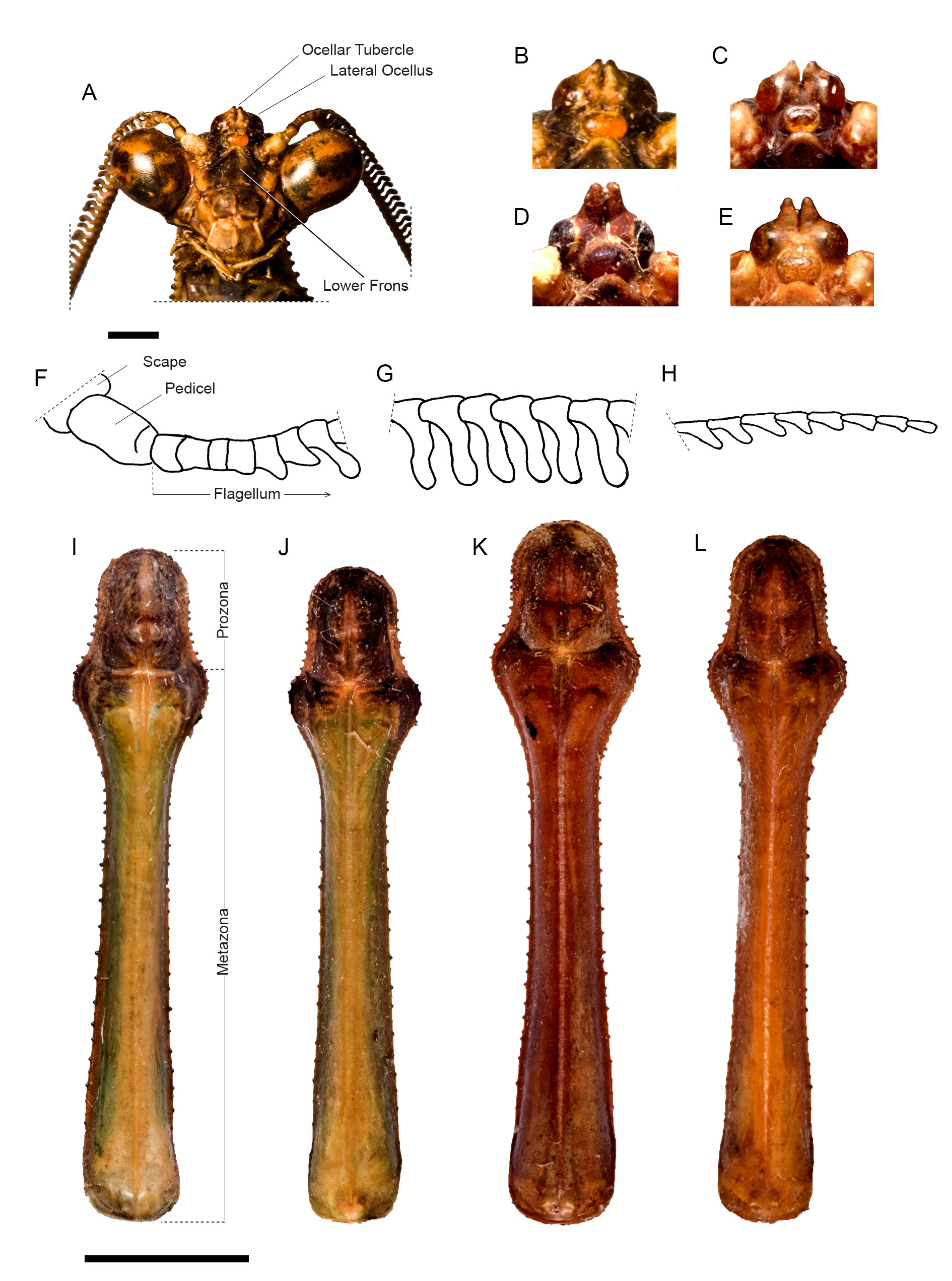
HEAD. Eyes rounded ( Fig. 3A View Fig ). Vertex flat, higher than imaginary line connecting dorsal margin of compound eyes. Juxtaocular bulges flat, aligned to vertex. Ocellar tubercles ( Fig. 3A View Fig ) bearing two poorly developed conical projections, one over each lateral ocellus, only scarcely spaced (variation of this character across examined specimens is shown in Fig. 3 View Fig B–E). Central ocellus elliptical, lateral ocelli rounded. Antennae ( Fig. 3 View Fig F–H) with scape and pedicel light brown, rest of antennae dark brown, proximal-most antennomeres cylindrical, eventually turning strongly asymmetrical, conferring antennae with a pectiniform appearance, distal-most antennomeres more or less filiform. Lower frons ( Fig. 3A View Fig ) sub-pentagonal, wider than high and with upper margin arcuate, surface smooth, concave and medially darkened. Maxillary palps light brown. Inner margin of labial palpi dark, basal-most segment with dark spots.
THORAX. Pronotum ( Fig. 3I View Fig ) elongated, metazona triangular in cross-section. Supracoxal dilation moderately pronounced and broadly rounded; ratio metazona / prozona = 4.12 (variation across specimens 4.5–3.75). Distal margin of prozona uniformly curved, margins with small, spine-like, blunt tubercles, denser along prozona than along metazona and mostly absent proximally. Dorsal surface of metazona keeled along its midline (keel more pronounced proximally). Pronotum predominantly green, except for darkened prozone and lateral margins of metazona. Variation in pronotal size and shape across examined specimens shown in Fig. 3 View Fig J–L.
PROTHORACIC LEGS. Forecoxae triangular in cross-section; ventral margin pale green, except for a small and distally positioned dark spot on its anterior aspect, and a larger preapical spot posteriorly; dorsal margin bearing five spine-like, darkened tubercles interleaved with smaller, paler ones; anterior aspect of forecoxae light colored, darked apically, rest of structure dark brown. Spination formula: F =4DS/14AvS/4PvS; T =14AvS/8(R)–10(L)PvS. Forefemora light brown, three-banded, with a small dark spot near trochanter; dorsal margin of forefemora slightly sinuous; discoidal spines I, II and III mostly pale with darkened apex, spine IV entirely dark; anteroventral spines II, IV, VI, X and XII slightly reclined and entirely dark, spine XV larger than the others, entirely dark and not curved; remaining spines smaller, pale and with darkened apex; genicular spines developed and present on both sides of femora; tibial spur groove located in proximal ¼ of femora. Foretibiae light brown, dorsally three-banded.
WINGS. Forewings ( Fig. 2C View Fig ) surpass abdomen by ¼ of its length in resting position. Costal area distally tapering, membrane opaque and predominantly green, with a yellowish longitudinal strip along margin of costal vein; discoidal area entirely hyaline with yellowish to brownish longitudinal veins, apex slightly darkened, with more densely reticulate venation. Hindwings ( Fig. 2C View Fig ) hyaline and colorless, yellowish to brownish longitudinal veins, apex of discoidal area densely reticulated and dark brown.
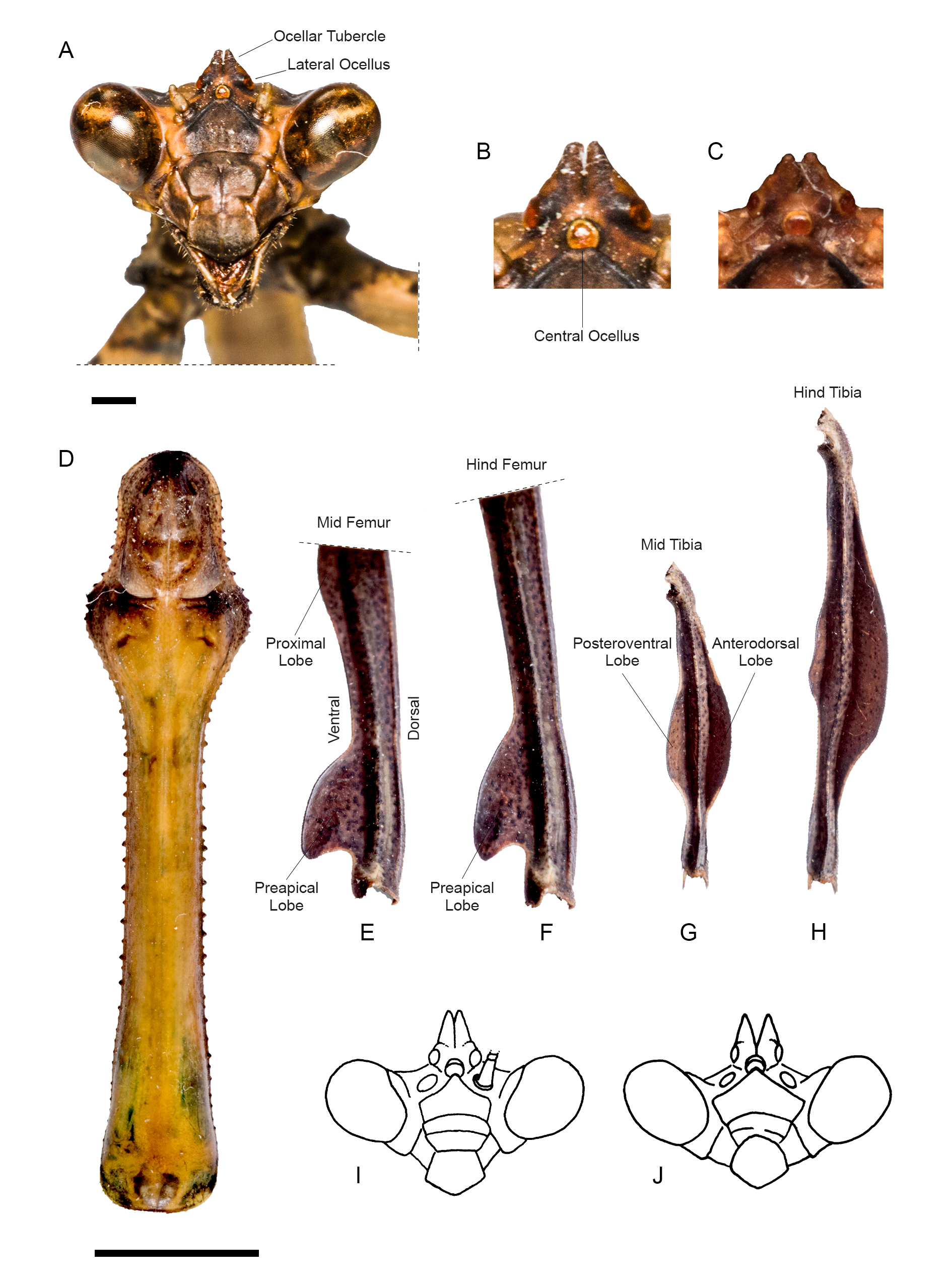
MESO- AND METATHORACIC LEGS. Mesothoracic femora ( Fig. 4A View Fig ) with a marked posteroventral keel that forms two evident lobes: a proximal, elongated but scarcely produced lobe, and a pre-apical, short and produced lobe whose shape resembles that of a shark’s dorsal fin (variation of this character across specimens can be seen in Fig. 4 View Fig B–D). Metathoracic femora ( Fig. 4E View Fig ) with a marked posteroventral keel that forms a single, preapical lobe, also shaped like a shark’s dorsal fin (variation of this character across specimens can be seen in Fig. 4F View Fig ). Mesothoracic tibiae ( Fig. 4G View Fig ) with two medial lobes: a produced, anterodorsal lobe almost as long as tibia itself, and an anteroventral one, almost equally produced but clearly shorter (variation of this character across specimens can be seen in Fig. 4 View Fig H–J). Metathoracic tibiae ( Fig. 4K View Fig ) with two elongated lobes, anterodorsal one longer and wider than anteroventral one, and also more prominent than its homologue on mesothoracic tibiae (variation of this character across specimens can be seen in Fig. 4 View Fig L–M).
ABDOMEN. Slightly compressed dorsoventrally, widest between segments III–V. Tergites I–III with a whitish coloration, remaining tergites darkened. Supraanal plate triangular in shape, wider than longer, apex medially notched, forming two small, lateral lobes. Cerci cylindrical and elongated, not surpassing apex of subgenital plate, last cercomere conical.
GENITALIA (note: holotype was not dissected; the following description corresponds to genital structures of paratypes). Left phallomere ( Fig. 5 View Fig A–E): sclerite L4B longer than wide, its left margin projects anteriorly; anterior process (afa) glabrous, sinuous, basal half broad but tapering distally, apex more strongly sclerotized, with sharp, pointy apex; posteromesal lobe (loa) elongated and sinuous, glabrous, lacking projections; posterior process (Paa) elongated, slightly curved left, apex curved anteriorly. Ventral phallomere ( Fig. 5 View Fig F–I): sclerite L4A roughly oval, proximal left margin slightly sinuous and strongly sclerotized, forming a small, medial projection followed by a membranous notch; posterior process (Pda) elongated, apex strongly scletorized and tapering distally, uniformly curved right, although its distal third curves anteriorly. Right phallomere ( Fig. 5 View Fig J–N): roughly triangular, distal margin folded anteriorly; anterior apodeme (an) of sclerite R3 elongated, distally broadened, bearing a small, basal process near its articulation to main posterior lobe (fda), the latter with a relatively short and broad dextral extension (bm); process anteromesal to Pia (Pva) elongated and finger-like, slightly curved ventrally, strongly sclerotized, with a moderately truncated apex; process posterolateral to Pva (Pia) elongated and well sclerotized.
Female (allotype; MNRJ-ENT6-28442)
HABITUS. Live specimen (allotype) in Fig. 2B View Fig ; pinned specimen (allotype) in Fig. 2D. View Fig
MEASUREMENTS. See Table 1 View Table 1 (specimen JB01).
HEAD. Eyes rounded ( Fig. 6A View Fig ). Vertex flat, higher than imaginary line connecting dorsal margin of compound eyes. Parietal suture darkened, juxtaocular bulges flat and aligned to vertex. Ocellar tubercles ( Fig. 6B View Fig ) bearing two poorly developed conical projections, one over each lateral ocellus and barely spaced (variation of this character across specimens can be seen in Fig. 6C View Fig ). Ocelli rounded, lateral ocelli twice as large as central ocellus. Antenna filiform, scape and pedicel light brown, flagellomeres dark brown. Lower frons sub-pentagonal, wider than high, smooth, medially darkened, upper margin arcuate, smooth and concave. Maxillary palps light brown; inner margin of labial palpi darkened, basalmost segment with dark spots.
THORAX. Pronotum elongated ( Fig. 6D View Fig ), metazona triangular in cross-section. Supracoxal dilatation moderately pronounced and broadly rounded; ratio metazona / prozona = 3.65 (additional paratype female = 3.78). Distal margins of prozona uniformly curved, margins with small, spine-like, blunt tubercles, denser along prozona than along metazona and mostly absent proximally. Dorsal surface of metazona keeled along its midline (keel more pronounced proximally). Pronotum predominantly green, except for darkened prozone and lateral margins of metazona.
PROTHORACIC LEGS. Forecoxae triangular in cross-section, ventral margin pale yellowish brown, except for small dark spot on its anterior end, and a larger, preapical one on its posterior aspect; dorsal margin with nine spine-like, darkened tubercles interleaved with smaller, paler ones; color patterning in general similar to that of males. Forefemora light brown, three-banded, and with a small spot in anterior region of each femur near trochanter. Dorsal margin of forefemora slightly sinuous. Spination formula of forelegs: F =4DS/15AvS/4PvS; T=15(R)–16(L)AvS/13(R)–11(L)PvS. Discoidal spines I, II and III pale with dark spots at their base and apex, spine IV entirely dark. Anteroventral spines II, IV, VI, VIII and X slightly reclined, spines I–XII entirely black, XIII and XIV black laterally and at apex, XV with black base and apex, spine XV largest, straight and darkened at its base and its tip; posteroventral spines black at their base and tips; a well developed genicular spine on each sides of femora; tibial spine groove located in proximal ¼ of femora. Foretibiae light brown, dorsally three-banded.
WINGS. Forewings ( Fig. 2D View Fig ) surpass abdomen by ¼ of their length in resting position. Forewings with membrane of costal area opaque and predominantñy green, with a yellowish longitudinal strip along margin of costal vein, distal-most portion of costal vein and membrane around radial vein darkened; discoidal area with green and opaque membrane, longitudinal veins mostly yellow, veins densely reticulated distally, stigma with a darkly pigmented spot; anal area small, mostly opaque, veins and membrane withish. Hindwing with narrow costal area, tapering distally and partially opaque, proximal half with membrane and veins whitish, although cells become dark brown distally; discoidal area largely hyaline with yellowish veins, proximal region of membrane whitish, distal portion smoky brown, opaque and heavily reticulated; anal area largely hyaline, veins and proximal area of membrane whitish, distal portion below discoidal area faint brown.
MESO- AND METATHORACIC LEGS. Mesothoracic femora ( Fig. 6E View Fig ) with a marked posteroventral keel that forms two evident lobes: a proximal one that is elongated but scarcely produced, and a pre-apical one that is short and produced, shaped like a shark’s dorsal fin. Metathoracic femora ( Fig. 6F View Fig ) with a marked posteroventral keel that forms a single preapical lobe, also shaped like a shark’s dorsal fin. Mesothoracic tibiae ( Fig. 6G View Fig ) with two medial lobes: a produced, anterodorsal one, almost as long as tibia itself, and an anteroventral one, almost equally produced but clearly shorter. Metathoracic tibiae ( Fig. 6H View Fig ) with two elongated lobes, anterodorsal one longer and wider than anteroventral one, and also more prominent than its homologue on mesothoracic tibiae.
ABDOMEN. Fusiform, slightly flattened dorsoventrally, widest between segments III–V, brownish with some contrasting spots in middle of each tergite. Cerci elongated, surpassing apex of subgenital plate, cercomeres cylindrical.
Differential diagnosis
Svenson et al. (2015) listed all species of Vates they considered valid, totaling 13 spp. All these species are herein discussed in relation to our new species. The males of V. phoenix sp. nov. share with those of V. biplagiata Sjöstedt, 1930 , V. luxuriosa Beier, 1958 , V. amazonica (Westwood, 1889) , V. pectinicornis (Stål, 1877) , V. foliata (Lichtenstein, 1802) and V. lobata ( Fabricius, 1798) the asymmetrical, s-shaped antennomeres, whereas the females share with those of V. serraticornis Stål, 1877 , V. festae Giglio-Tos, 1914 , V. weyrauchi Beier, 1958 , and likely also V. boliviana Giglio-Tos, 1914 (which remains known from males only) the yellowish white tinge of the hindwing membrane. None of the preceding species has both males with s-shaped antennomeres and females with yellowish white hindwings. Therefore, V. phoenix sp. nov. possesses a unique combination of character states unknown in other species of Vates , making its identification straightforward. In the absence of either one of the sexes for effective comparisons, the reduced ocellar tubercles of V. phoenix sp. nov. represent a distinct character state for the species, common to both sexes, and unique among members of the genus (compare, for instance, with Fig. 6 View Fig I–J). Our new species can also be easily distinguished from V. pectinata Saussure, 1871 and V. chopardi (Deeleman-Reinhold, 1957) for lacking the dorsal, preapical lobe of the forefemora, a distinct character shared by the latter two species ( Fig. 7 View Fig ) — which Roy (2012) suggested as likely synonyms. Additionally, we were unable to compare V. phoenix sp. nov. with the type specimen of Vates lobata ( Fabricius, 1798) . This species was originally described from “Cajennae” (= Cayenne, French Guiana) as Mantis lobata ( Fabricius 1798) in the ‘Supplementum’ to ‘Entomologica Systematica’ — not to be confused with Mantis lobata Fabricius, 1781 , a synonym of Harpagomantis tricolor (Linnaeus, 1758) (Galinthiadidae) sensu Beier (1934). Examination of the Banks collection (JR) housed by the Natural History Museum, London (containing a sizable portion of Fabricius types) did reveal the presence of Mantis lobata Fabricius, 1781 , though no specimen attributable to Mantis lobata Fabricius, 1798 (i.e., Vates ) was found there. The taxa that Fabricius (1798) described from “Cajennae” in his ‘Supplementum’ were owned by Louis Augustin Guillaume Bosc d’Antic (N. Kristensen, pers. com. 2011), a French naturalist whose insect collection, in part studied by Fabricius, dispersed across European natural history institutions after his death in 1828 ( Notton 2007). A good portion of this material eventually made it to Paris and Geneva; however, examination of these and other European collections also containing some of Fabricius’ types, such as the Zoological Museum in Copenhagen (N. Kristensen, pers. com. 2011) and the Hunterian Museum in Glasgow (online catalogue: http://collections.gla.ac.uk/) did not turn up any specimen attributable to the type of Mantis lobata Fabricius, 1798 . This specimen is most likely lost. Regardless of the fate of the type specimen, the new species can be distinguished from Vates lobata on the basis of its distribution, as French Guiana and Rio de Janeiro are biogeographically distant and unrelated regions that do not share any praying mantis species. Finally, Vates obscura Toledo Piza, 1983 , listed as valid in Svenson et al. (2015), had already been synonymized with V. biplagiata in an earlier publication ( Agudelo & Rivera 2015), and thus we conclusively remove this species from the Vates checklist.
Systematic remarks
Morphological comparison of male genitalia provided insights on the affinities of the new species. The only species whose male genital structures are known are Vates chopardi ( Lombardo 2000: figs 25–27), and V. biplagiata and V. festae ( Medellín & Salazar 2011: fig. 14). Morphological comparisons between V. phoenix sp. nov. and V. biplagiata genitalia showed that both species share a strong, proximally bent afa, whereas the accentuated sigmoidal shape of the same can also be observed in V. chopardi , though in the latter the afa is not bent to the same degree as in V. biplagiata . Differences in genital structures are more accentuated between V. phoenix sp. nov. and V. festae , the latter with a much straighter afa and, and a shortened and robust Pda on the ventral phallomere. Coincidently, the phylogeny of Vatini proposed in Svenson et al (2015) recovered three main clades within Vates : one containing V. chopardi , sister to another clade comprised of V. festae and V. weyrauchi , altogether sister to a more diverse clade containing V. biplagiata . Our analysis of external morphology and existing reports of male genital structures thus suggests a closer affinity to members of this latter clade, which in Svenson et al. (2015) also included V. luxuriosa , V. amazonica , V. pectinicornis and two additional, unidentified species. Further phylogenetic studies are necessary to test this hypothesis and resolve evolutionary affinities with all members of Vates .
With the description of our new species and the clarification of previous records, the following species are considered valid: 1) V. amazonica (Westwood, 1889) ; 2) V. biplagiata Sjöstedt, 1930 ; 3) V. boliviana Giglio-Tos, 1914 ; 4) V. chopardi (Deeleman-Reinhold, 1957) ; 5) V. festae Giglio-Tos, 1914 ; 6) V. foliata (Lichtenstein, 1802) ; 7) V. lobata ( Fabricius, 1798) ; 8) V. luxuriosa Beier, 1958 ; 9) V. pectinata Saussure, 1871 ; 10) V. pectinicornis (Stål, 1877) ; 11) V. serraticornis Stål, 1877 ; 12) V. weyrauchi Beier, 1958 ; 13) V. phoenix Rivera et al. sp. nov. The possible synonymy between V. pectinata and V. chopardi , as suggested by Roy (2012), is pending confirmation.
Natural history and behavior
Rehn (1935) noted that species of Vates were difficult to come across in nature. Things have not changed much since Rehn’s times, as there still is very little information in the literature regarding the biology of Vates . Below we present natural history information of Vates spp resulting from our own observations and literature accounts, and summarize this information to place it in the context of our new species.
Neither nymphs nor adults of V. phoenix sp. nov. were ever found in their natural habitat. Unlike members of other sympatric genera, all broadly sampled across Rio de Janeiro by Projeto Mantis’ research team, we were unable to locate specimens of Vates in any manually sampled plant formations in the three years that this project spanned. This suggest that Vates spp. likely prefer higher layers within the vegetation as perching and hunting grounds, thus making their collection at ground level difficult. In fact, Dantas et al. (2008) reported Vates spp. flying to canopy light traps 45 m above the ground near Manaus, Brazil. Interestingly, the latter study reported collecting a few females, thus evidencing enhanced flying capabilities of this sex in Vates . Behavioral observations conducted on the only wild-caught female indeed showed that this sex is able to sustain controlled gliding for short distances while moving across perching sites. Our anatomical examinations of V. phoenix sp. nov. revealed that females have relatively larger ocelli compared to other non-Vatini females, a pattern consistent with flying capabilities in female praying mantises and other insects ( Battiston et al. 2018). Further, reduced sexual dimorphism in wing size and shape is evident in the examined specimens (e.g., Fig. 2 View Fig C–D). This likely explains why females of Vates are sometimes lured to light traps, as shown by Dantas et al. (2008) and corroborated by some of us through field work (JR, pers. obs.). Collecting efforts using this sampling method could eventually result in collecting additional female specimens of V. phoenix sp. nov.
Angatuba (São Paulo) and Reserva Ecológica de Guapiaçu (Rio de Janeiro) represent the westerneasternmost limits of Vates phoenix sp. nov. based on the available data. Unfortunately, it is difficult to determine the extent of the original distribution of V. phoenix sp. nov. and other sympatric species, as the original Atlantic Rainforest circumscribed by these localities is now heavily altered by urban development and farming, thus affecting this and likely other praying mantis species ( Rodrigues & Cancello 2013). Sampled localities showed that V. phoenix sp. nov. ranges from 7 to 600 m. Distributional records compiled from the literature revealed that several species of Vates are found at mid- to higher elevations. For instance, the dominant species along the oriental slopes of the Peruvian / Ecuadorian Andes and neighbouring lowlands are V. weyrauchi (100– 1000 m), V. luxuriosa (400– 1000 m), V. biplagiata (180 – 2900 m) and V. festae (1000 – 1700 m) ( Lombardo & Agabiti 2001; Rivera & Vergara-Cobián 2017), with the latter also found on the eastern slopes of the central and oriental cordilleras of central Colombia (450– 2400 m) ( Medellín & Salazar 2011; Svenson et al. 2015) and in the vicinity of Manaus, Brazil (ca 90 m) ( Dantas et al. 2008). The relatively narrow vertical distribution of V. phoenix sp. nov. is comparable to that of V. amazonica and V. chopardi , both predominantly lowland species mostly found below 500 m, the former across the Amazon basin and the latter along the Mexican Pacific and Atlantic lowlands ( Svenson et al. 2015). All this information suggests that several species of Vates have a broad altitudinal range (e.g., V. biplagiata ), and that the genus has a tendency to be more diverse at higher elevations. However, distributional records across the Amazonian lowlands are still scarce for most species and more surveys are necessary to infer patterns of vertical distribution across the genus.
Reared specimens preferred perching clinging upside-down, often keeping their raptorial forelegs stretched forward to form a 90º angle relative to the body ( Fig. 8 View Fig ). This posture was assumed either de novo or enhanced in response to a nearby observer the insect possibly perceived as a potential threat. Robinson (1969) reported a similar behavior in Pseudovates chlorophaea (Blanchard, 1836) (cited as Phyllovates chlorophaea therein) in Panama, regarding it as a combination of leaf and stick mimicry. More efforts are necessary to unveil further aspects of the natural history of Vates spp, and of Vatini in general.
Table 1. Standard body measurements of Vates phoenix sp. nov. (in mm). Foretibial measurement D1 does not include the tibial spur, whereas D2 does. Measurements under FR01 correspond to the holotype and JB01 to the allotype.Abbreviations: FR = Fazenda Recanto; JB = Jardim Botânico do Rio de Janeiro; RG = Reserva Ecológica do Guapiaçu; CO = Corcovado; JU = Jussaral; AN =Angatuba. Standard measurements sensu Brannoch et al. (2017).
| Character | FR01 | JB01 | FR02 | FR03 | RG01 | RG02 | CO | JU01 | JU02 | JU03 | JU04 | JU05 | JU06 | AN | JB02 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | ♂ | ♀ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♀ |
| Body length | 64.0 | 67.5 | 66.0 | 66.0 | 64.0 | 64.5 | 64.0 | 67.0 | 68.0 | 64.0 | 68.5 | 67.5 | 68.5 | 66.0 | 65.0 |
| Head width | 5.0 | 6.9 | 6.0 | 6.0 | 6.0 | 6.0 | 5.5 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.5 | 6.0 | 7.0 |
| Head length | 2.5 | 3.0 | 2.5 | 2.5 | 2.5 | 2.5 | 2.0 | 2.0 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.3 | 3.0 |
| Lower frons width | 1.5 | 2.5 | 1.5 | 1.5 | 1.5 | 1.5 | damaged | 1.5 | 1.0 | 1.0 | 2.0 | 1.5 | 1.0 | 1.0 | 2.5 |
| Lower frons length | 1.0 | 1.4 | 1.0 | 1.0 | 1.0 | 1.0 | 1.5 | 1.0 | 1.0 | 0.6 | 1.0 | 1.0 | 1.0 | 1.0 | 2.5 |
| Pronotal length | 20.5 | 23.2 | 22.5 | 21.3 | 20 | 19 | 19.5 | 21.5 | 20.5 | 19.5 | 21.5 | 21.5 | 21.5 | 21 | 23 |
| Prozone | 4.0 | 4.2 | 4.5 | 4.0 | 4.0 | 4.0 | 3.5 | 3.5 | 3.5 | 3.5 | 4.0 | 4.0 | 4.0 | 4.0 | 4.1 |
| Metazone | 16.5 | 19 | 18.0 | 17.3 | 16.0 | 15.0 | 16.0 | 18.0 | 17.0 | 16.0 | 17.5 | 17.5 | 17.5 | 17.0 | 18.9 |
| Pronotal width | 4.0 | 5.2 | 4.0 | 4.5 | 4.0 | 4.0 | 4.0 | 4.5 | 4.0 | 4.0 | 4.3 | 4.0 | 4.0 | 4.0 | 5.0 |
| Ratio metazone/ prozone | 4.12 | 3.65 | 4.50 | 3.85 | 4.00 | 3.75 | 4.00 | 4.00 | 4,25 | 4.00 | 04.05 | 4.37 | 4.37 | 4.37 | 3.78 |
| Forewing length | 42.0 | 40.3 | 44.0 | 42.3 | 41.0 | 43.5 | 40.0 | 43.0 | 43.5 | 43.0 | 44.0 | 43.0 | 43.5 | 40.5 | 40.0 |
| Forewing width | 9.0 | 10.0 | 9.0 | 8.5 | 8.0 | 8.5 | - | - | - | - | - | - | - | - | - |
| Hindwing length | 37.5 | 35.2 | 38.0 | 37.6 | 39.5 | 39.0 | - | - | - | - | - | - | - | - | - |
| Hindwing width | 17.0 | 18.6 | 16.0 | 17.5 | 16.0 | 16.0 | - | - | - | - | - | - | - | - | - |
| Forecoxal length | 11.0 | 14.4 | 11.0 | 11.0 | 11.0 | 11.0 | 11.0 | 11.0 | 11.0 | 10.6 | 11.0 | 11.5 | 11.5 | 11.5 | 13.5 |
| Forecoxal width | 1.5 | 2.5 | 1.5 | 1.6 | 1.5 | 1.5 | 2.0 | 2.0 | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | 1.6 | 2.0 |
| Forefemoral length | 14.0 | 15.3 | 14.5 | 12.5 | 12.5 | 11.5 | 12.0 | 13.0 | 13.0 | 12.3 | 12.6 | 13.0 | 12.6 | 12.5 | 15.0 |
| Forefemoral width | 1.6 | 2,3 | 2.0 | 1.6 | 1.6 | 1.6 | 1.5 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 1.6 | 3.0 |
| Foretibial length (D1) | 5.0 | 6.0 | 5.5 | 5.0 | 4.6 | 4.5 | 4.5 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 4.5 | 5.5 |
| Foretibial length (D2) | 7.0 | 9.0 | 7.5 | 7.33 | 7.0 | 7.0 | 7.0 | 7.5 | 7.0 | 7.0 | 7.5 | 7.0 | 7.33 | 7.33 | 8.0 |
| Mesocoxa | 4.0 | 6.5 | 5.5 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.5 | 4.0 | 4.5 | 4.5 | 5.0 | 4.5 | 6.0 |
| Mesofemur | 11.0 | 12 | 11.0 | 11.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 11.0 | 11.0 | 11.0 | 11.0 | 12.3 |
| Mesotibia | 8.3 | 9,6 | 9.0 | 8.6 | 9.0 | 8.0 | 8.0 | 8.0 | 8.5 | 9.0 | 9.0 | 8.5 | 8.6 | 8.6 | 10.0 |
| Mesotarsus | 6.0 | 7,7 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 5.0 | 6.0 | 6.5 | 6.0 | 5.5 | 6.5 | 6.0 | 7.0 |
| Metacoxa length | 4.0 | 5.7 | 4.0 | 4.5 | 4.5 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.5 | 4.5 | 5.0 | 4.5 | 5.5 |
| Metafemur length | 13.5 | 14,3 | 15.0 | 13.5 | 13.5 | 13.0 | 12.0 | 13.0 | 12.0 | 12.0 | 14.0 | 13.5 | 13.66 | 13.6 | 15.3 |
| Metatibia length | 11.5 | 14,3 | 11.5 | 12.0 | 12.0 | 11.5 | 11.0 | 12.0 | 11.5 | 12.0 | 12.5 | 12.0 | 12.0 | 11.6 | 13.0 |
| Metatarsus length | 6.0 | 8.5 | 7.0 | 7.5 | 7.0 | 7.5 | 6.5 | 7.0 | 7.0 | 7.5 | 8.5 | 8.0 | 8.5 | 8.5 | 8.0 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.