Cyclogethes tibialis Liu, Huang & Audisio, 2024
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5406.2.8 |
|
publication LSID |
lsid:zoobank.org:pub:16586E3F-0FB0-4D96-A069-C93A86ACCB37 |
|
DOI |
https://doi.org/10.5281/zenodo.10618470 |
|
persistent identifier |
https://treatment.plazi.org/id/03EE8791-F25B-D817-0897-D17CA6698864 |
|
treatment provided by |
Plazi |
|
scientific name |
Cyclogethes tibialis Liu, Huang & Audisio |
| status |
sp. nov. |
Cyclogethes tibialis Liu, Huang & Audisio , sp. nov.
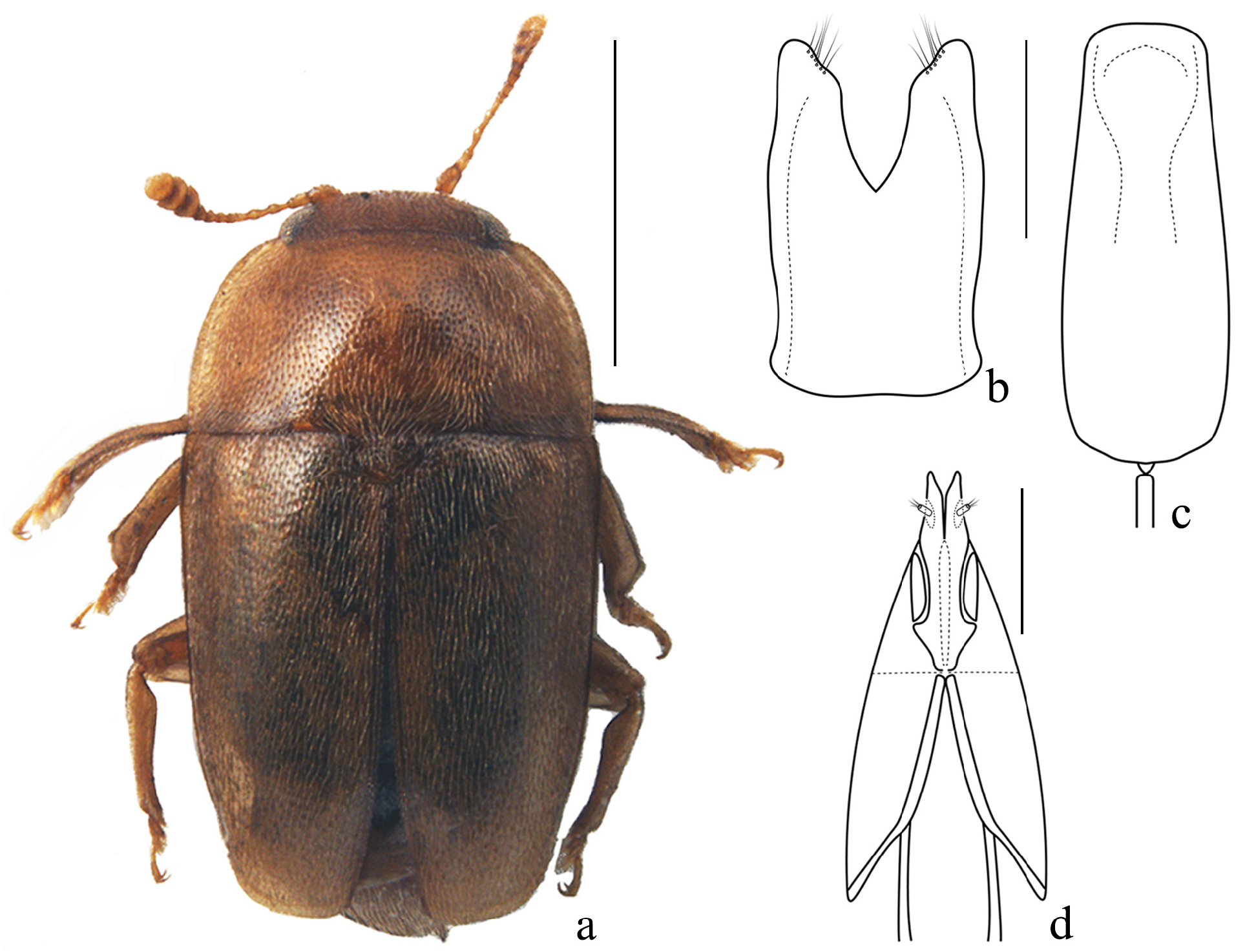
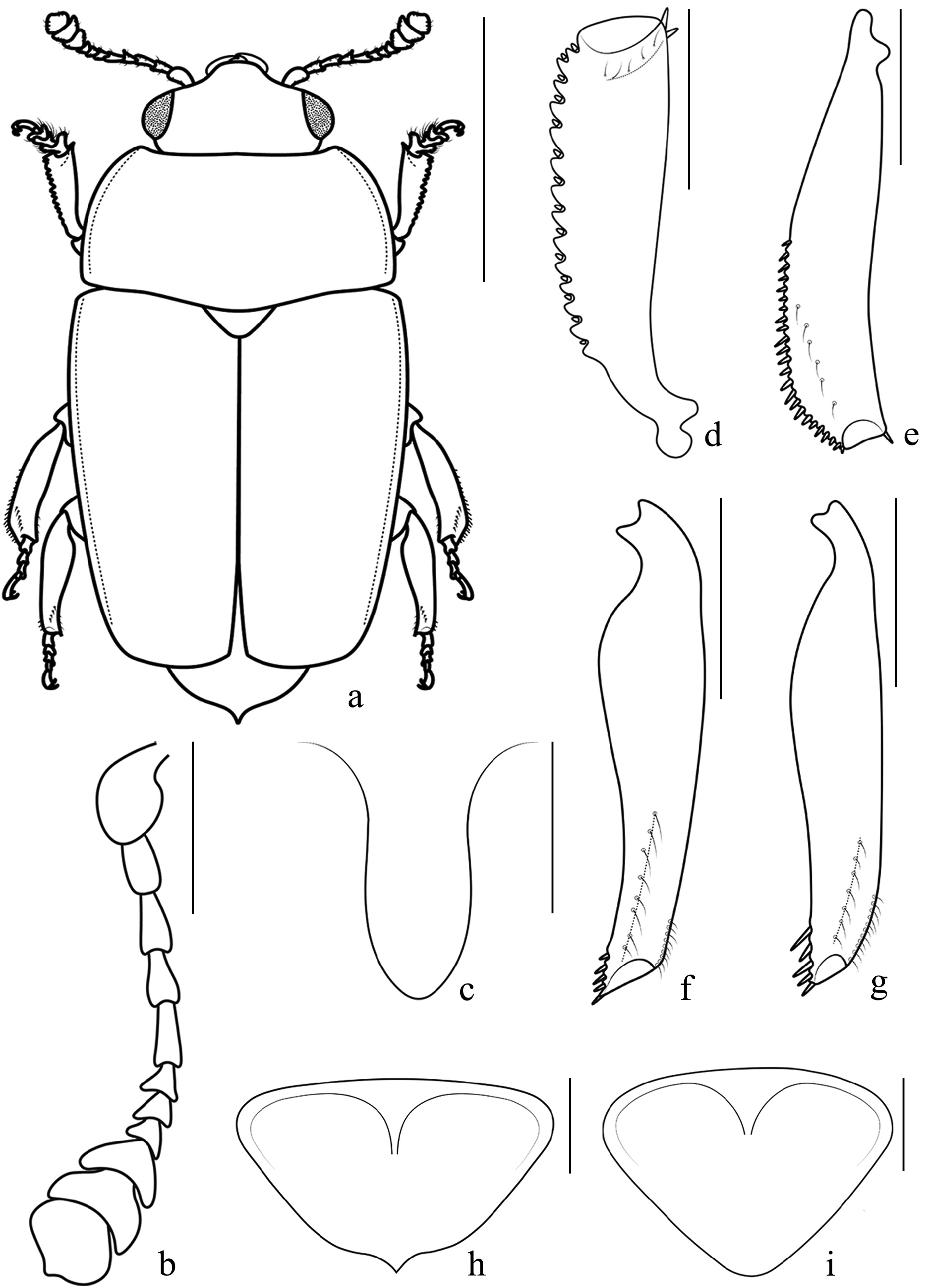
Figs 1a–d View FIGURE 1 ; 2a–i View FIGURE 2
Diagnosis
Narrowly elongate, scarcely transversely convex, densely, and rather deeply punctuated, uniformly testaceous-orange; vaguely recalling the Oriental Cyclogethes abnormis Kirejtshuk, 1979 (from NE India, N Indochina, and SW China), but easily differentiated by the peculiarly shaped (narrow and markedly sinuate) hind tibiae in both sexes, the more elongate body shape, the male pygidium markedly pointed distad, and the different male and female genitalia.
Etymology. The specific epithet is derived from the unusually narrow shape of the metatibiae in both sexes, markedly sinuate along their inner edge even in females.
Material examined
Holotype. CHINA ♂; Yunnan: Yuxi, Xinping YizuDaizu Autonomous County, Gasa Town, Ailao Mountain Nature Reserve, ca. 1965 m a.s.l., ca. 24.003372°N, 101.547364°E, 09.iv.2017, Chen & He lgt, beating yellow and incompletely flowered corymbs of Pseudognaphalium affine (D. Don) Anderberg ( Asteraceae , subfamily Gnaphalieae ; Fig. 3 View FIGURE 3 ) ( NWAU).
Paratypes. CHINA 3 ♀♀; Yunnan: same data as holotype ( NWAU, CAR-MZUR).
Description (male holotype)
Measurements. Body length 2.5 mm, width 1.3 mm.
Colour. Body uniformly testaceous-orange ( Fig. 1a View FIGURE 1 ), without distinctly paler or darker areas, including peripheral margins of pronotum, only with vaguely infuscate discal portions of elytra; legs and antennae uniformly yellowishorange, testaceous, including antennal club.
Body shape. Body moderately elongate, scarcely transversely convex ( Figs 1a View FIGURE 1 , 2a View FIGURE 2 ).
Dorsal punctation. Surface moderately densely, coarsely, and deeply punctate (spaces between pronotal and elytral punctures ca. 1–1.5× their diameter), with barely shagreened and rather shining interspaces; elytra without traces of transverse strigose sculpturing.
Prothorax. Pronotum with trapezoidal shape, widely arcuated lateral sides, maximum width nearly at its posterior angles ( Figs 1a View FIGURE 1 , 2a View FIGURE 2 ). Pubescence sparse, golden-whitish, rather long, and distinct, each individual seta nearly as long as 5 th antennomere, slightly longer only along posterior base. Notosternal sutures distinct, slightly darker than the remaining part of prosternum, only very slightly raised. Median flat portion of the prosternal process narrowly rounded distad, rather parallel-sided, its maximum width nearly in the middle of its whole length, here nearly as wide as antennal club ( Fig. 2c View FIGURE 2 ).
Elytra. Elytra rather parallel-sided, ratio LELY/WELY ≈ 1.02. Pubescence sparse, golden-whitish, rather long and distinct, each individual seta nearly as long as 5 th antennomere.
Metathorax. Metaventrite with a rather shallow, nearly pentagonal impression, occupying its posterior half, deeper in its longitudinal middle. Last abdominal ventrite bearing a couple of rather large proximal semicircular impressions, their diameter nearly as 1.3× the maximum diameter of each eye.
Pygidium. Proximal base of pygidium with normal, “ V ” shaped carina in the middle, directed backwards ( Fig. 2h View FIGURE 2 ). Pygidium distinctly pointed distad, with small, acute, and evident apical projection directed backwards ( Figs 2a, h View FIGURE 2 ).
Legs. Front tibiae rather wide, triangular, ratio PTLE/PTWI ≈ 0.3, with a series of very small and short tegumental teeth ( Fig. 2d View FIGURE 2 ); front tarsi nearly as wide as front tibiae, and nearly as wide as the length of the 1 st antennomere ( Fig. 2a View FIGURE 2 ); middle tibiae rather narrow, moderately sinuated ( Fig. 2e View FIGURE 2 ), their outer edge with a normal and distinct series of small dense spinules. Hind tibiae narrow (ratio MTLE/MTWI ≈ 4.5), peculiarly shaped, along their inner side strongly modified and narrowed in their posterior half, and markedly sinuate ( Figs 1a View FIGURE 1 , 2a, f View FIGURE 2 ), their outer edge without a series of spinules (only with a short series of very small hairs distad), without any similitude with any other hitherto known member of its genus.
Antennae. Antennal club elongate, symmetrical, without evident sexual differentiation ( Fig. 2b View FIGURE 2 ).
Male genitalia. Distinctly shaped, rather small, with elongate and subparallel-sided tegmen ( Fig. 1b View FIGURE 1 ), and roundly pointed apex of each paramere; ratio DTIN/LETE ≈ 0.42–0.43, the excision’s inner margins with a small pre-distal gibbosity; ratio LETE/WITE ≈ 1.70. Aedeagal median lobe peculiarly shaped, slightly narrowed in its distal third, with maximum width near its proximal third; subtruncate but weakly rounded distad ( Fig. 1c View FIGURE 1 ); ratio LEAE/WIAE ≈ 2.6.
Female. Front tibiae moderately wide, slender, distinctly narrower than in males, front tarsi slightly narrower than front tibiae (ratio WFTA/LFTA ≈ 0.25). Middle tibiae nearly as in males. Hind tibiae slightly narrower than in males (MTLE/MTWI ≈ 4.7), but along their inner side markedly sinuate, nearly as in males ( Fig. 2g View FIGURE 2 ). Pygidium regularly rounded distad, without any pointed projection ( Fig. 2i View FIGURE 2 ). Metaventrite in females almost flat, without distinct impression, in the middle only with a barely impressed longitudinal line. Ovipositor rather small and scarcely sclerotized, not darkened towards the moderately pointed and distinctly divaricated distal apex, exhibiting moderately long styli, inserted rather far from the apex ( Fig. 1d View FIGURE 1 ). Ratio STLE/DSIA ≈ 0.42; ratio STLE/CGOW ≈ 0.23; ratio GONL/CGOW ≈ 3.1. Ratio OVPL/GONL ≈ 2.15.
Variation. Body size 2.2–2.5 mm (length) and 1.2–1.3 mm (width). Antennae without evident differences among sexes ( Fig. 2b View FIGURE 2 ).
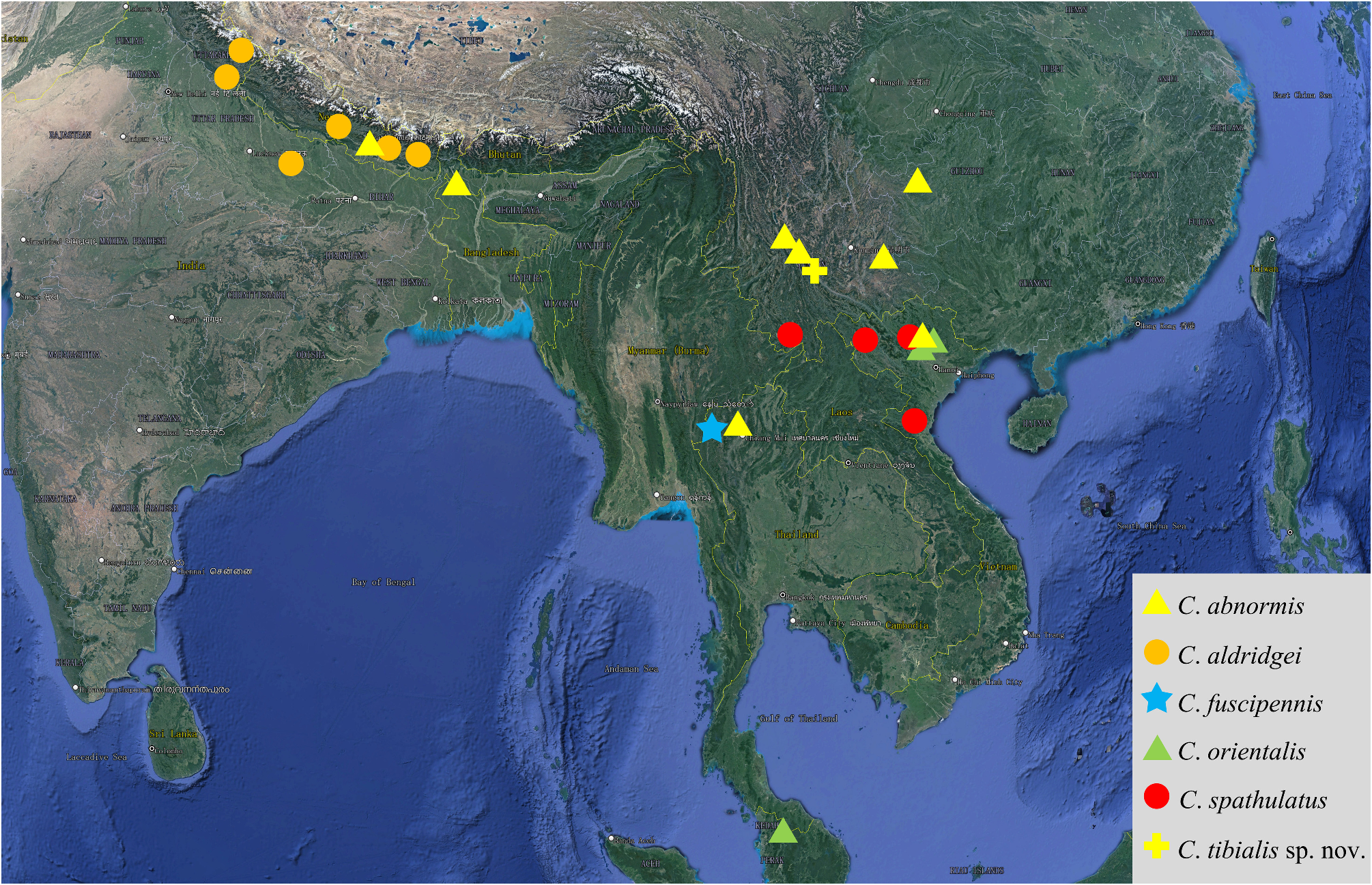
Distribution
SW China (Yunnan) ( Fig. 4 View FIGURE 4 ).
Phenology
The thus far available specimens of the new species were collected in early-middle April, which likely indicates adult local activity in Spring (maybe from early April to late June or early July).
Hostplants
As above reported, the four known adult specimens of the new species were all collected in Yunnan on the yellow buds (inflorescence corymbs in prefloration) of a member of the family Asteraceae , subfamily Gnaphalieae , Pseudognaphalium affine (D. Don) Anderberg ( Fig. 3 View FIGURE 3 ). This observation alone does not allow to demonstrate any possible larval host-plant relationship, although the high females/males sex ratio (3:1) could make a true larval trophic relationship slightly more likely. As discussed in the Introduction, the larval hostplants of all members of the genus Cyclogethes are unknown. However, Audisio et al. (2009b, 2015b) have hypothesized a probably close phylogenetic relationship between Oriental Cyclogethes and the southern and eastern African genus Tarchonanthogethes Audisio & Cline, 2009 . Being all known members of the latter genus associated as larvae with male inflorescences of small trees and shrubs of the family Asteraceae (subfam. Carduoideae, tribe Tarchonantheae : Panero & Funk, 2002; Funk et al. 2009; Audisio et al. 2009b, 2015b), we cannot exclude that similar insect-hostplant relationships could link members of Cyclogethes with inflorescences of some Oriental Asteraceae . In this scenario, the best candidates could perhaps be represented by members of some Asian genera that include shrublets and small trees, such as, e.g., Gymnanthemum Cass. , Monosis DC. , or Vernonia Schreber , but also Pseudognaphalium Kirp. (in the tribe Gnaphalieae , not far from Tarchonantheae : Funk et al. 2009; Mandel et al. 2019) could certainly be taken in account. New insights and further contributions also on phylogenetic relationships between African and Oriental Asteraceae ( Mandel et al., 2019) could maybe help in identification of the true hostplants. However, further field research in SW China has been programmed in the next few years, to discover with certainty (i.e., finding the larval stages) the hostplants of Cyclogethes species.
Habitat
Locality data indicates that the new species could prefer sparsely forested and bushy areas or thickets, in rocky and rather steep habitats, at the edge of subtropical mountain forest (ca. 2000 m a.s.l.). The other known species of Cyclogethes have been all collected in tropical and subtropical forest habitats, thickets, and forest clearings ( Table 1 View TABLE 1 ), at altitudes comprised between ca. 400 and ca. 2800 m a.s.l. ( Kirejtshuk 1979, 1980; Kirejtshuk & Kabakov 1997; Jelínek 2000b; Audisio et al. 2009b; Liu 2019).
Taxonomic remarks
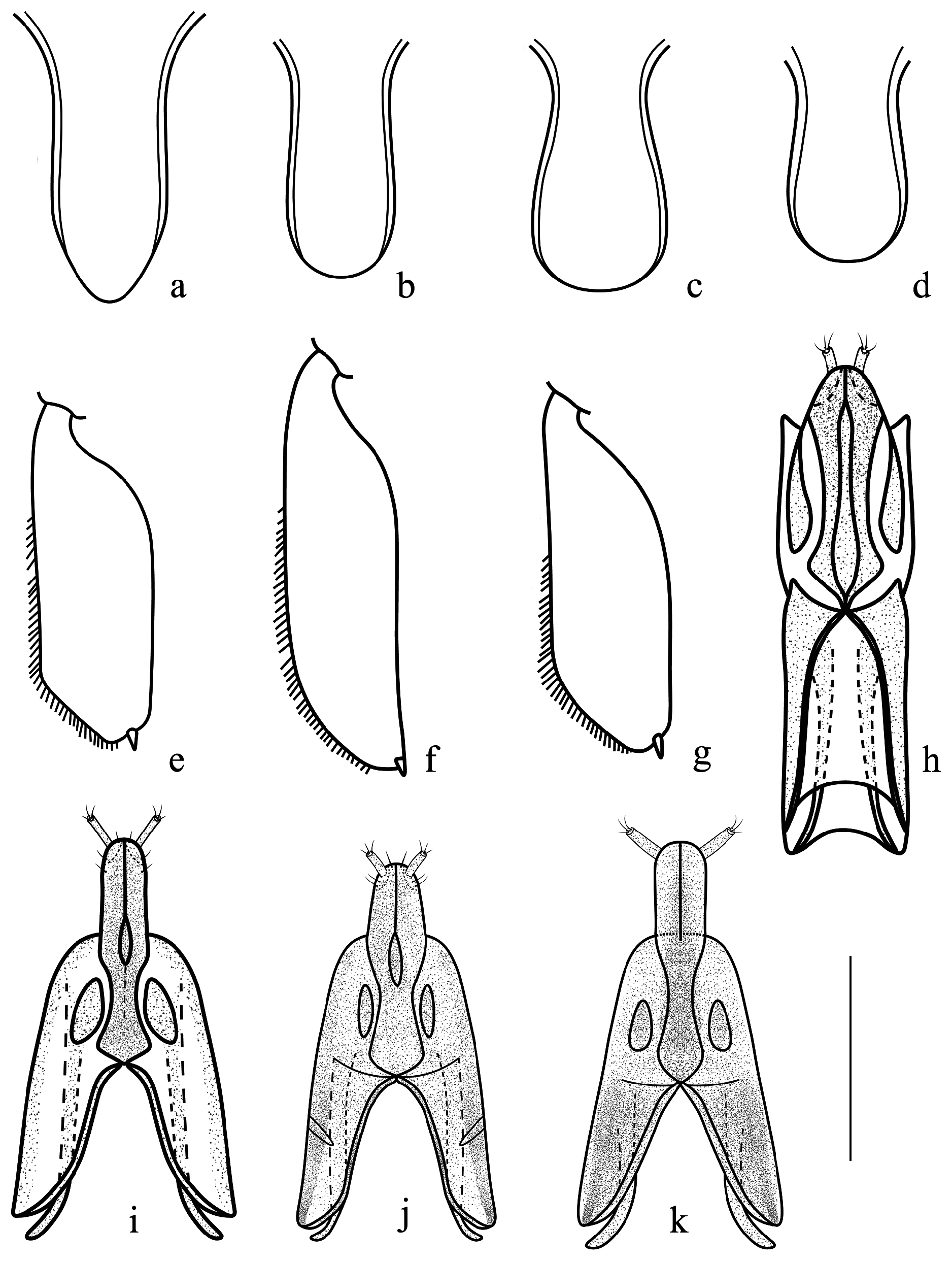
As reported above, this new species certainly belongs to the Cyclogethes abnormis species-group, being vaguely related to Cyclogethes abnormis from NE India, N Indochina, and SW China, and to C. aldridgei from N India and Nepal ( Table 1 View TABLE 1 ). The new species is otherwise unmistakable due to the very peculiar shape of the male and female hind tibiae, the larger and more elongated body shape, the deeper and coarser dorsal punctation, the dorsal elytral surface without any trace of transversal strigosity, the acutely pointed apex of pygidium in males, as well as by its distinctly shaped male genitalia and ovipositor. However, the peculiar shape of the ovipositor of the new species, markedly bifid distad, and with minute pre-distal styli ( Fig. 1d View FIGURE 1 ), probably suggests a rather isolated position for the new species, the more related taxa of the Cyclogethes abnormis species-group sharing, in fact, a simply rounded apex of gonostyloids, with larger distal styli (Fig. 43 in Kirejtshuk 1979, Fig. 6k View FIGURE 6 herein; Fig. 84 in Kirejtshuk 1980; Fig. 6h View FIGURE 6 herein).
| NWAU |
North-West Agricultural University |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |