Hylomyscus kerbispeterhansi, Demos & Agwanda & Hickerson, 2014
|
publication ID |
https://doi.org/ 10.1644/13-MAMM-A-268 |
|
DOI |
https://doi.org/10.5281/zenodo.7814438 |
|
persistent identifier |
https://treatment.plazi.org/id/03E887D9-FFE2-FFE9-FF68-FC5E9745D6DB |
|
treatment provided by |
Jucosta |
|
scientific name |
Hylomyscus kerbispeterhansi |
| status |
sp. nov. |
Hylomyscus kerbispeterhansi View in CoL View at ENA , new species
Praomys ( Hylomyscus) denniae vulcanorum: Bishop, 1979:528 ; part.
Hylomyscus denniae: Clausnitzer and Kityo, 2001:101 View in CoL ; part.
Holotype.—Field Museum of Natural History, Division of Mammals catalogue number 210017 (field number TCD 2924), collected 25 July 2010 by T. Demos during a faunal survey of the Mau Escarpment, Kenya. The specimen, an adult female, was fixed in 10% formalin solution and subsequently transferred to 70% ethanol. The skull was extracted from the fluid specimen and cleaned and is in excellent condition ( Fig. 2 View FIG ). The specimen has full adult dentition and fusion of basisphenoid–basioccipital sutures. External measurements were made in the field and include: total length, 234 mm; head and body length, 95 mm; tail length, 139 mm; hind-foot length, 20 mm; ear length, 19 mm; and body mass, 24.5 g. This specimen was included in all morphometric and molecular analyses. An aliquot of muscle tissue was taken from the specimen in the field and preserved in dimethylsulfoxide prior to final cryogenic storage at —180°C at FMNH.
Type locality.— Kenya, Rift Valley Province, Narok District, Mau Escarpment, 15.5 km N, 16.4 km E Bomet, 0.64170°S, 35.49104°E, 2,350 m elevation.
Paratypes.—Three males, FMNH 209997 , 210001 , and 210018 , and 3 females, FMNH 210000 , 210015 , and 210040 , all collected during 22–25 July 2010 at the type locality are deposited in the FMNH. All specimens were fixed in 10% formalin and subsequently preserved in 70% ethanol, with crania extracted and cleaned. Muscle tissue samples were preserved in dimethylsulfoxide in the field and subsequently stored at —180°C at FMNH. Identification of all paratypes was confirmed by Cytb molecular sequence data.
Additional specimens.—To morphometrically delineate the new taxon and study its genetic variability numerous other specimens of Hylomyscus from the H. anselli and H. denniae groups from the type locality and other East African localities were included in our analyses. Representative skulls for comparison are provided in Fig. 3 View FIG . Summary statistics for univariate mensural variables are provided in Table 1 View TABLE . These additional specimens examined are listed in Appendix I.
Diagnosis.—A member of the H. anselli group as characterized by the absence of 1 pectoral pair of teats (mammae total = 6), conspicuously shorter incisive foramina, and larger subsquamosal fenestrae with more slender hamular process relative to members of the H. denniae group ( denniae , endorobae , and vulcanorum —sensu Carleton and Stanley 2005). Subsquamosal fenestrae are larger than in other members of the H. anselli group. H. kerbispeterhansi is intermediate in the majority of cranial measurements between relatively smaller H. arcimontensis and relatively larger H. anselli including zygomatic breadth, length of nasals, postpalatal length, width of M1, and crown length of upper toothrow ( Table 1 View TABLE ).
Comparisons.— Hylomyscus kerbispeterhansi is larger than H. arcimontensis in key cranial measures: zygomatic breadth, length of incisive foramina, length of diastema, and length of auditory bullae ( Table 1 View TABLE ). Differences with H. anselli are more subtle, although H. kerbispeterhansi is generally smaller in most cranial and external measures. The crown length of upper toothrow of H. kerbispeterhansi is shorter and its range is nonoverlapping with H. anselli (3.6–3.93 mm versus 3.97– 4.51 mm). H. kerbispeterhansi has smaller and more anteriorly situated posterior palatal foramina that are located between M1 and M2, which are not visible in ventral view, but can be seen in lateral or oblique views. In H. anselli the posterior palatal foramina are much larger, are readily visible in ventral view, and continue posteriorly across the anterior one-third of the M2 ( Fig. 3 View FIG ); in H. kerbispeterhansi , the frontoparietal suture is very broadly U-shaped, whereas in H. anselli this suture is more Vshaped; in H. kerbispeterhansi , the subsquamosal fenestra is larger and the postglenoid foramen is more arched compared to in H. anselli ; in H. kerbispeterhansi , the zygomatic plate is more orthogonal (less sinuous) than in H. anselli (in the new species, the plate may even be slanted slightly backward, whereas in H. anselli , it often bulges forward with a more rounded profile); incisive foramina are wider in center in H. kerbispeterhansi compared to H. anselli and mesopterygoid fossa is more constricted medially in H. kerbispeterhansi in comparison to H. anselli , which lacks any narrowing along fossa length.
Description.—Pelage soft and fine in texture, rather long (8– 10 mm over middle rump) and close-lying. Dorsal body hairs dark slate gray over most of their length with medium brownish red tips; pelage grades to lighter rufous brown along flanks; guard hairs blackish brown and distinctly longer than body fur of lower dorsum. Ventral pelage appears whitish gray; basal one-half medium gray and distal one-half white. Young specimens are blackish gray. Tail distinctly longer than head and body (TAIL = 148% ± 11.2% of HB); color dark chocolate brown; caudal scales finely textured and hairs short, about 1.5–2 annuli in length; tail appears naked over most of its length, fine hairs becoming longer and brighter toward the tip. Pinnae dark brown. Hind feet short and narrow; 5 digits; plantar surface naked, with 6 well-formed pads. In the forefeet, the 5th digit is long, approximately equal to digits 2–4. Tops of forefeet and hind feet are covered with short, light brown hair; claws are covered by white tufts of hair. There are 6 mammae distributed as 1 axial and 2 inguinal pairs.
The skull is delicate overall as in other members of the H. anselli group (Carleton and Stanley 2005) and characterized by small size, short rostrum, and thin zygomatic plates. The braincase is smooth and distinctly arched over parietals. Rostral processes of premaxillaries terminate approximately equal with the rear border of the nasals; interorbital breadth relatively narrow, lacking supraorbital ridging or beading (as in H. aeta ). Zygomatic plate medium in width; dorsal notch is shallow. Hard palate smooth, slightly concave dorsally; posterior palatal foramina lie between the rear of M1 and front of M2. Ectotympanic bullae moderately inflated for the genus. Upper incisors enamel face yellow-orange. Upper molar row about as long as the hard palate and toothrows parallel. Incisive foramina moderately long (LIF = 71% ± 3.5% of LD), posteriorly terminating just short of or equal to the anterior root of the 1st molars; foramina broad over their anterior portion, more strongly constricted over posterior one-half. Mesopterygoid fossa is constricted medially in comparison to both H. anselli and H. arcimontensis .
Phylogenetically, the new species is distinguished as reciprocally monophyletic from other members of the H. anselli group based on mitochondrial Cytb and 3 autosomal intron (ABHD, ACOX2, and GAD2) sequence data. H. kerbispeterhansi is strongly supported as sister to H. anselli ( H. arcimontensis ( H. kerbispeterhansi , H. anselli )).
Ecology and reproduction.—All specimens of H. kerbispeterhansi were collected in forested habitats above 2,300 m in elevation. Activity is strictly nocturnal because all were collected during morning trap checks (approximately 0700–0800 h). The arboreal habits of this climbing mouse are not well documented, although arboreal habits have been documented for other Hylomyscus species ( Stanley et al. 1998; Nicolas et al. 2008). Although most commonly captured in traps placed on the ground, 22 of 152 specimens were captured in traps set 1–2 m off the ground on vines and tree limbs. Specimens from Mau Escarpment were collected during 2010 from a variety of forested habitats including well-drained closed-canopy montane forest (2,350 m), selectively logged montane forest (2,300 –2,360 m), montane secondary forest with bracken (2,320 m), and in dense undergrowth alongside montane forest streams (2,210 –2,320 m). H. kerbispeterhansi is sympatric with H. endorobae in the western Mau Escarpment and they were found to be syntopic in several traplines where both species were captured in the same station. Hylomyscus species were the 3rd most abundant rodent species in the Mau forest after Praomys jacksoni and Lophuromys aquilus based on 1,505 trap-nights. No specimens of H. endoroba e were collected during 2,400 trap-nights of collecting in Mt. Elgon or 700 trap-nights in Cherangani Hills, strongly suggesting that the ranges of H. kerbispeterhansi and H. endorobae overlap only in the Mau Escarpment in what may be a zone of secondary contact. Thus, H. kerbispeterhansi is limited to Kenya’s western montane blocks (Mt. Elgon and Cherangani Hills), whereas the eastern montane blocks (Mt. Kenya and Aberdare Mts.) house only H. endorobae . Extensive surveys by TCD, BA, and colleagues of the aforementioned east Kenyan montane forests have uncovered no presence of H. kerbispeterhansi east of the Kenyan Rift Valley. Surveys of the Cherangani Hills and Mt. Elgon in 2011 found H. kerbispeterhansi in primary montane forest (2,740 m, Cherangani Hills), selectively logged and secondary montane forest (2,520 –2,770 m, both mountains), and in mixed bamboo– Hagenia forest (2,540 m, Mt. Elgon). In both of these disjunct montane forests H. kerbispeterhansi was the most abundant rodent species, comprising 44% and 42% of terrestrial rodents recorded, respectively. An extensive survey at a 2nd higher-elevation camp on Mt. Elgon (3,000 –3,180 m) did not record any Hylomyscus specimens during 1,138 trapnights in a variety of microhabitats that included upper montane forest–alpine grassland mosaics, Hagenia groves, and gallery forest along the Kimothon River.
Among 8 adult females examined for reproductive condition 3 were pregnant, with the number of embryos in a single uterine horn ranging from 1 to 4 and the number for both horns combined ranging from 3 to 5, with an average litter size of 4. The embryos averaged 14.7 mm in crown–rump length (range = 12–18 mm). Among 9 adult males examined, testes averaged 14 mm in length (range = 8–19 mm) and 6.9 mm in width (range = 4–9 mm).
Etymology.—The new species is named in honor of Julian Kerbis Peterhans, in recognition of his significant contributions to current knowledge on African small mammals, his generosity in sharing data and specimens resulting from his extensive fieldwork, as well as his ongoing efforts in promoting African conservation and education.
Distribution.— Hylomyscus kerbispeterhansi is currently known from tropical montane forests of the Mau Escarpment, Cherangani Hills, and Mt. Elgon in Kenya, with an elevational range of 2,320 –2,740 m. Although not presently recorded from Ugandan slopes of Mt. Elgon, we presume it is distributed in these montane forests, which are continuous with those on the Kenyan slopes of Mt. Elgon.
Nomenclatural statement.—An LSID number was obtained for the new species ( Hylomyscus kerbispeterhansi ): urn:lsid:zoobank.org:pub:2E2A198B-70AB-49EF-98D5- 2CE0240002D4.
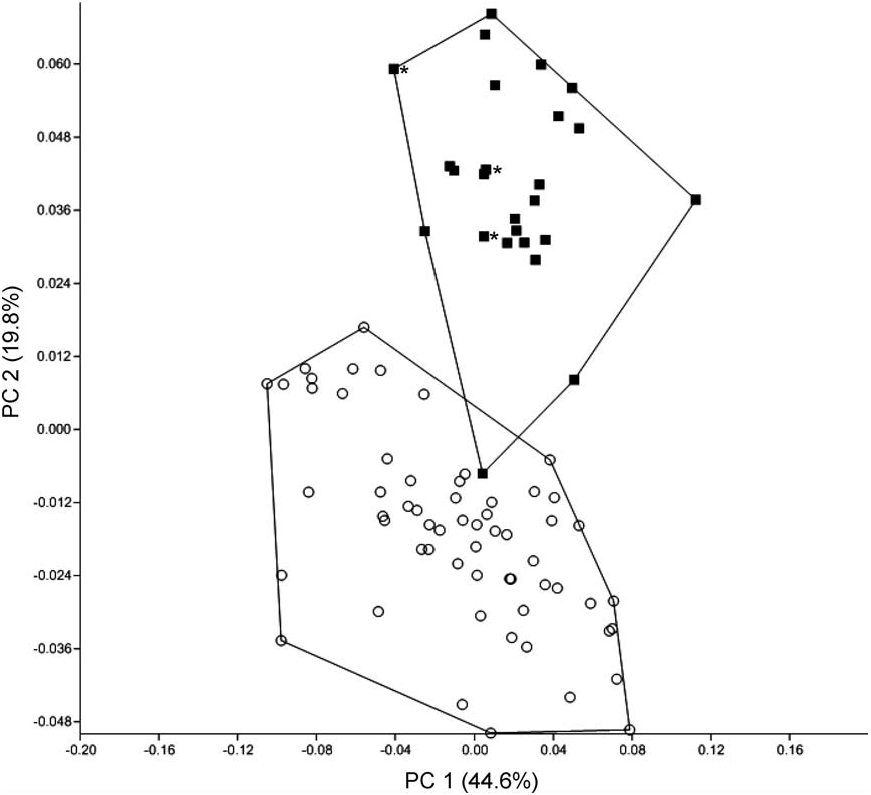
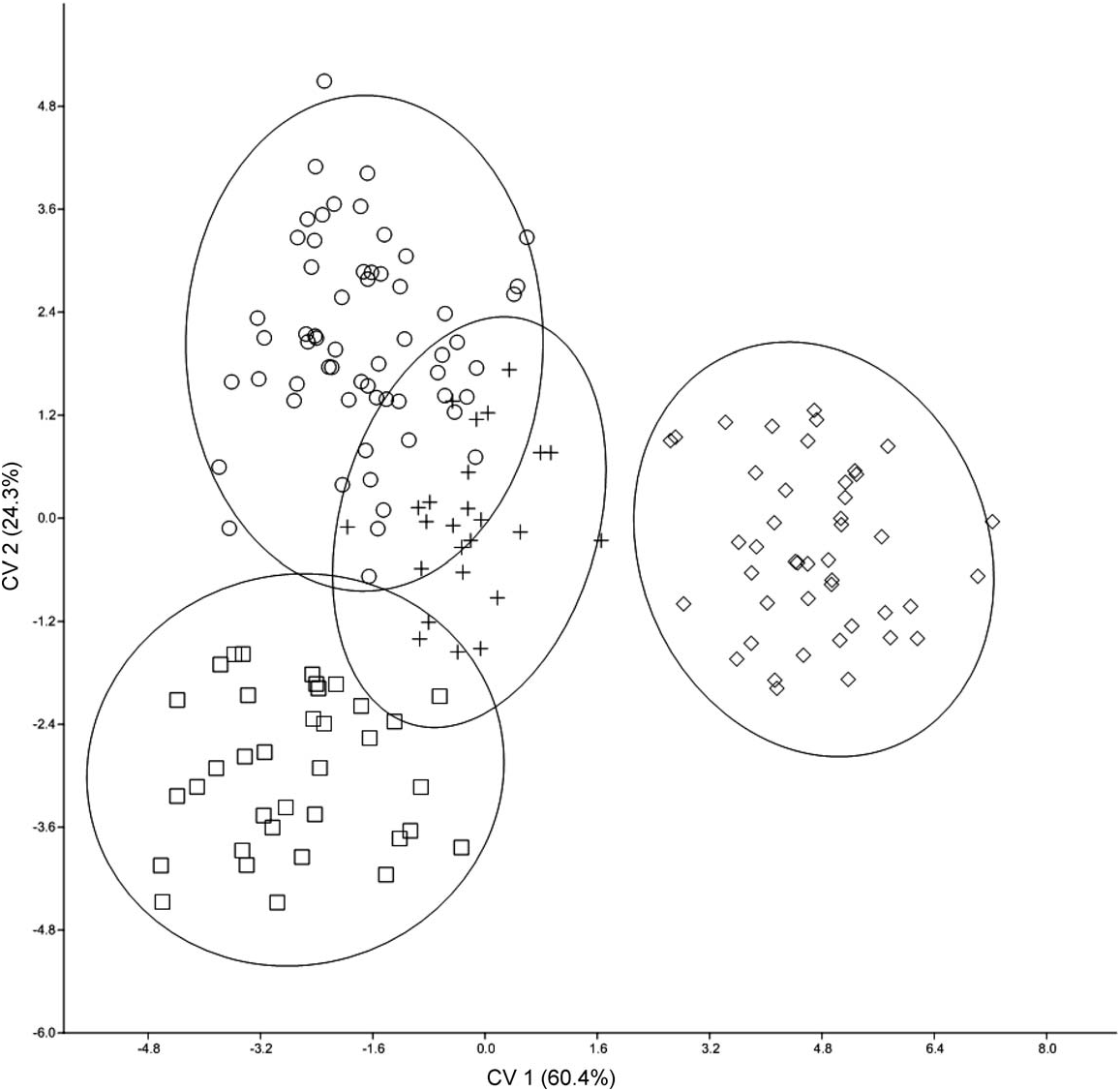
Morphometrics.—A principal component analysis was performed on 14 log-transformed craniodental variables for specimens of H. kerbispeterhansi and H. anselli with the 2 taxa occupying mostly discrete regions of multivariate space ( Fig. 4 View FIG ). Results of a discriminant function analysis of 14 logtransformed craniodental variables for specimens of H. kerbispeterhansi , H. anselli , H. arcimontensis , and H. endorobae are summarized in Fig. 5 View FIG . Multivariate ordinations performed on the 1st and 2nd canonical variates (CV1 and CV2) accounted for 85% of the cumulative proportion of variation for skull characters and showed well-defined morphometric structure with moderate overlap in multivariate space among specimens assigned to H. kerbispeterhansi , H. arcimontensis , and H. anselli , and no overlap with H. endorobae . CV1 discriminates populations of H. endorobae (within the H. denniae group) from members of the H. anselli group. The standardized coefficients for canonical variables matrix indicates LD is the most important negatively correlated variable and ONL, LIF, and CLM are the most negatively correlated variables on the CV1 axis ( Table 2 View TABLE ). CV2 discriminates among species assigned to the H. anselli group with specimens assigned to H. kerbispeterhansi having marginal overlap with H. anselli . On the CV2 axis ONL and PPL are the most negatively correlated variables and LD and CLM are the most positively correlated variables. ONL weights heavily on CV1 and distinguishes the larger crania of H. endorobae from the 3 members of the H. anselli clade. LD weights most heavily on CV2 and discriminates most among the 3 H. anselli group clades. The group centroids of the 4 species showed highly significant statistical differences (Wilks’ lambda = 0.007, F 42,440 = 45.1, P <0.0001). Squared Mahalanobis distances between species were 23.5 between H. kerbispeterhansi and H. anselli , 25.7 between H. arcimontensis and H. kerbispeterhansi , and 46.8 between H. kerbispeterhansi and H. endorobae . Dendrograms based on the Mahalanobis distance matrix and a corrected between-group genetic distance matrix using Cytb sequence data inferred the same topology and similar branch lengths for all 4 species ( Fig. 6 View FIG ).
When entered as unknowns in the 4-group discriminant function analysis and classified according to posterior probabilities of group membership, 1 individual from H. anselli and 1 individual from H. arcimontensis were assigned to the incorrect species in the classification table. All specimens of H. kerbispeterhansi were assigned to their appropriate taxonomic cluster.
Species-tree inference and Bayesian species delimitation.— The 3 autosomal introns we sequenced for H. denniae , H. anselli , and H. stella had between 31 and 78 segregating sites, whereas the mtDNA Cytb locus had 278 segregating sites. All 3 of the independent gene trees for the nuclear loci support populations of H. kerbispeterhansi from west-central Kenya as a reciprocally monophyletic group, distinct from species within the H. anselli and H. denniae species groups. The mtDNA gene tree strongly supports H. kerbispeterhansi as monophyletic with little divergence between populations from Mau Escarpment, the Cherangani Hills, and Mt. Elgon as distinct (Supporting Information S1, DOI: 10.1644/13-MAMM-A-268. S1). Mean between-group genetic distances (Kimura 2- parameter) for Cytb are 3.2% between H. kerbispeterhansi and H. anselli and 7.4% between H. kerbispeterhansi and H. arcimontensis ( Table 3 View TABLE ). Distances between species assigned to the H. anselli group and H. endorobae from the H. denniae group range from 15.3% to 16.0%. Additional Kimura 2- parameter genetic distances are given for 3 introns in Table 3 View TABLE .
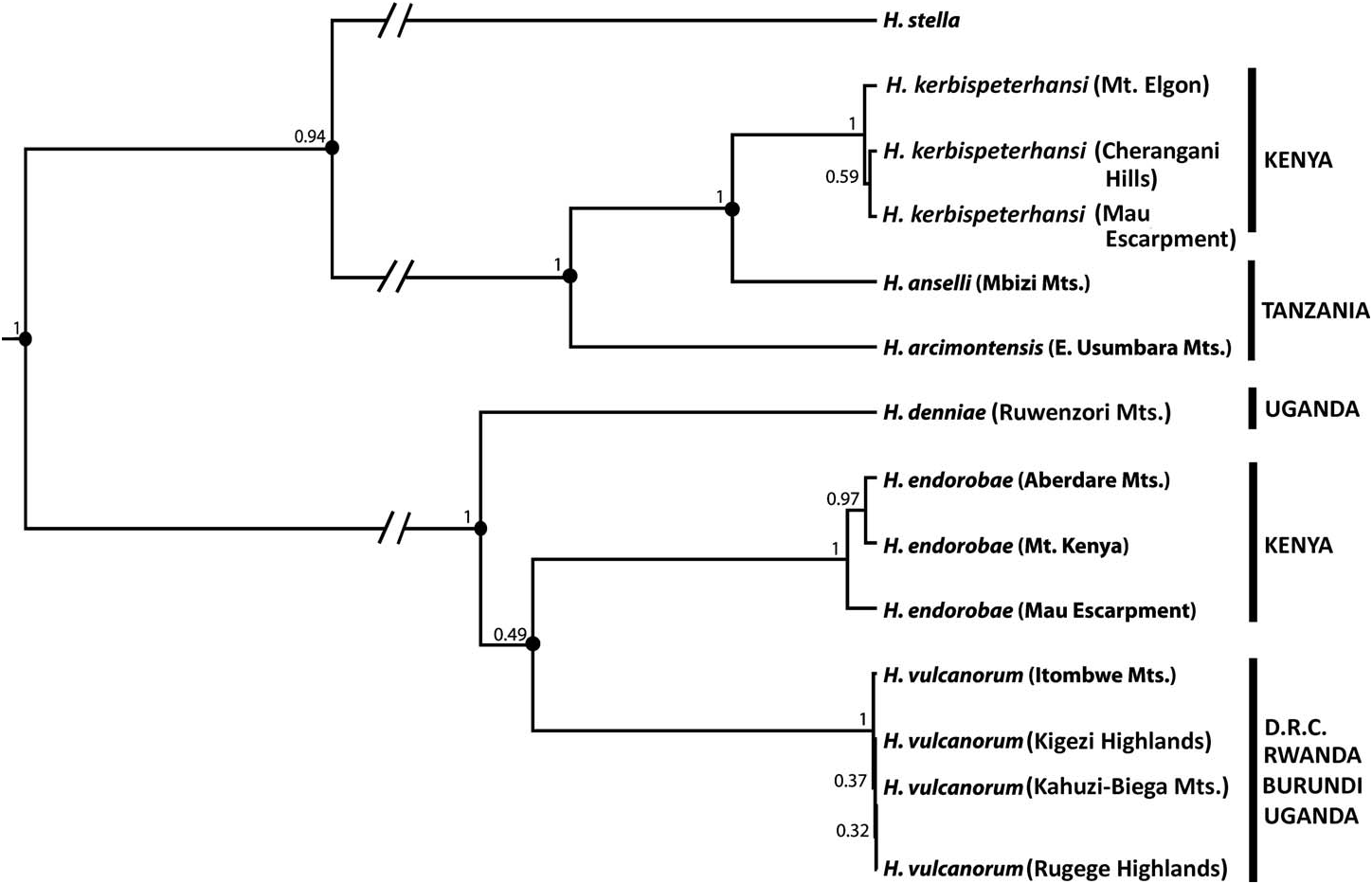
The *BEAST species-tree analysis strongly supports H. kerbispeterhansi as sister to H. anselli and H. anselli + H. kerbispeterhansi as sister to H. arcimontensis ( Fig. 7 View FIG ). The species tree also recovers those clades delimited as species on the basis of morphometric and morphological analyses in revisions of the H. anselli and H. denniae groups (Carleton and Stanley 2005; Carleton et al. 2006) with posterior probabilities of 100%. The topology of the 3 members of the H. denniae group is not well resolved, with <70% support for H. vulcanorum as sister to H. endorobae .
The Bayesian species delimitation results also are indicated on Fig. 7 View FIG and support the H. anselli group as comprising 3 species that includes the new species with speciation probabilities of 1.0 at relevant nodes. The 3 clades referred as species by Carleton and Stanley (2005) from the H. denniae group also are supported, with speciation probabilities of 1.0. We emphasize that these species delimitations were applied to taxa on the basis of populations assigned to individual mountain ranges (e.g., Itombwe Mountains, Cherangani Hills, and Mt. Kenya), and that we did not a priori designate species to the guide tree required by BPP at the species level for those clades distributed across multiple mountain ranges (e.g., H. endorobae distributed across 3 mountain ranges). We consider this to be a conservative approach because we are not biasing assignment of populations to species, but instead relying on statistical measures of support for lineages based on the results of the species-tree analysis (Leaché and Fujita 2010; Camargo et al. 2012). When populations were a priori assigned to species the same Bayesian species delimitations were recovered.
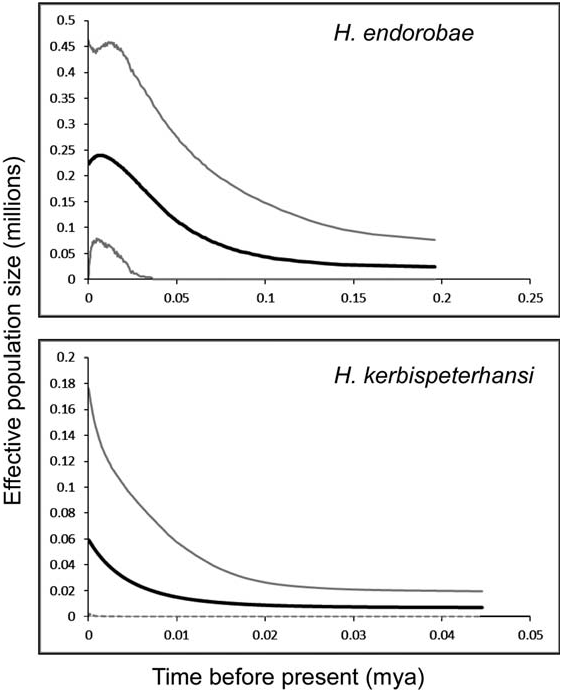
Historical demography.—Summary statistics from Cytb sequence data (1,119 base pairs) indicate a similar number of haplotypes (h) in H. kerbispeterhansi (h = 12) as in H. endorobae (h = 14). The numbers of individuals and localities also are similar between the 2 samples: H. kerbispeterhansi , n = 41 individuals from 4 unique localities on 3 mountains; and H. endorobae , n = 25 individuals from 5 unique localities on 3 mountains. Haplotype diversity was approximately equal for both taxa (Hd = 0.900 in H. kerbispeterhansi and Hd = 0.870 in H. endorobae ), whereas nucleotide diversity was low for both taxa with P = 0.0026 and 0.0019 for the respective species. Extended Bayesian skyline coalescent analyses, based on 5 nuclear intron loci and the Cytb locus, for populations across Kenya, support population and geographic expansion dating 10,000 –20,000 years ago for H. kerbispeterhansi and from ~ 100,000 years ago for H. endorobae , although with wide 95% highest posterior density intervals ( Fig. 8 View FIG ). Our sampling strategy of combining populations (demes) from across Kenya within each species for skyline analysis has been shown to minimize false inferences of demographic change ( Heller et al. 2013). This pattern is broadly coincident with Pleistocene forest refugial fragmentation during the last glacial maxima and subsequent refugial expansion after the last glacial maxima.
Results from a species-tree chronogram place the divergence between the H. anselli and H. denniae groups broadly within the Miocene (approximately 7.4 mya) and between H. anselli and H. kerbispeterhansi within the middle to late Pleistocene (approximately 0.83 mya [Supporting Information S2, DOI: 10.1644/13-MAMM-A-268.S2]). The split between H. anselli from southwestern Tanzania and H. kerbispeterhansi from the western Kenyan Highlands is broadly coincident with the intensification of global glacial climatic cycling and the intense aridification of Africa that commenced approximately 1 mya ( deMenocal 2004; Anhuf et al. 2006).
| TCD |
TCD |
| FMNH |
USA, Illinois, Chicago, Field Museum of Natural History (also used by Finnish Museum of Natural History) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hylomyscus kerbispeterhansi
| Demos, Terrence C., Agwanda, Bernard & Hickerson, Michael J. 2014 |
Hylomyscus kerbispeterhansi
| Demos & Agwanda & Hickerson 2014 |
Hylomyscus anselli
| : Carleton 2006: 310 |
Hylomyscus denniae
| : Clausnitzer and Kityo 2001: 101 |
Praomys ( Hylomyscus ) denniae vulcanorum:
| Bishop 1979: 528 |