Zoothamnium plumula Kahl, 1933
|
publication ID |
https://doi.org/ 10.5281/zenodo.278023 |
|
DOI |
https://doi.org/10.5281/zenodo.6190284 |
|
persistent identifier |
https://treatment.plazi.org/id/03E687ED-FF9B-8855-B3A6-8C40FAC0FD84 |
|
treatment provided by |
Plazi |
|
scientific name |
Zoothamnium plumula Kahl, 1933 |
| status |
|
Zoothamnium plumula Kahl, 1933
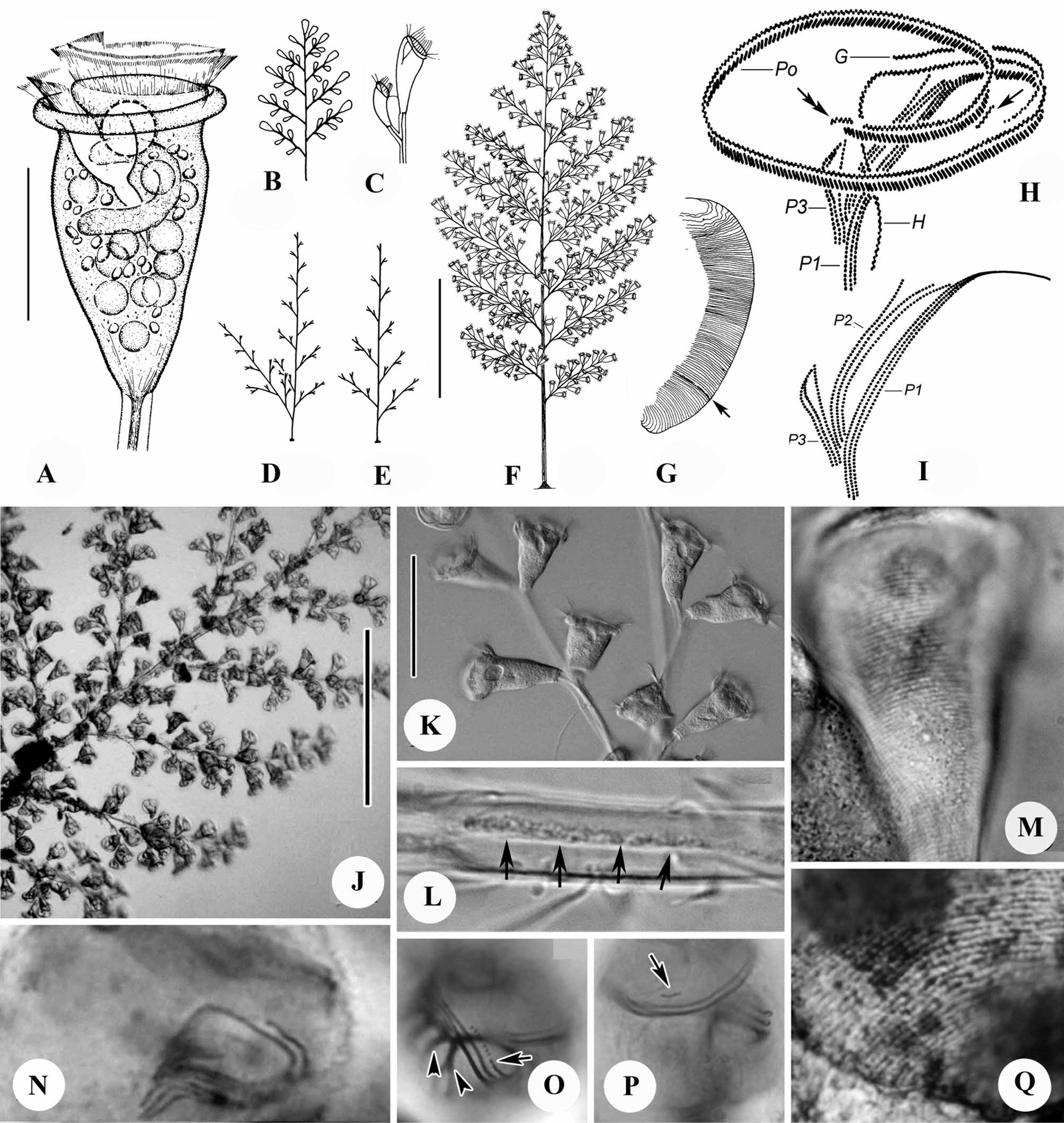
( Fig. 1 View FIGURE 1 ; Table 1)
Emended diagnosis. Marine Zoothamnium with colony up to 3 mm high; elongate, median primary stalk giving rise to secondary stalks in regular alternate series in single plane to create feather-shaped outline. Zooids conical to vase-shaped, measuring 50–75 × 35–45 µm in vivo; peristomial lip thick, without medial, circumferential infolding when expanded. Macronucleus C-shaped, transversely oriented, located in oral half of cell. Pellicular striations closely spaced and indistinct at lower magnifications; 50–70 silverlines lying between peristomial lip and trochal band and 20–30 between trochal band and scopula. P3 consists of three rows of kinetosomes that are equal in length; row 1 separated from rows 2 and 3 by gap in abstomal quarter and all three rows converging adstomally.
Redescription. Colony large, up to 3 mm tall and including over 500 zooids. Secondary stalks up to 800µm long, branching off elongate primary stalk in regular, alternate series in one plane; tertiary stalks with same pattern of branching, measuring less than 120µm in length ( Figs. 1 View FIGURE 1 B, D–F, J). Secondary stalks increasing slightly in length from most basal ones to medial region of colony and then decreasing in length toward tip of colony to create feather-shaped outline. Occasionally, most basal secondary stalk more highly developed and considerably longer than other secondary stalks ( Fig. 1 View FIGURE 1 D). Stalk with diameter of 16 µm in basal portion of primary stalk, narrowing progressively to diameter of 8 µm at distal ends of tertiary stalks; cortex of stalk colorless and transparent, with smooth surface and fine longitudinal striations in interior. Spasmoneme relatively more dense than cortex of stalk, with diameter of 5–6 µm in primary stalk, narrowing progressively to diameter of 2.5 µm at distal ends of tertiary stalks; band of mitochondria, visible as dark granules with diameter of 0.8 µm, winding along helical path just beneath surface of spasmoneme ( Fig. 1 View FIGURE 1 L). Colony relatively insensitive to stimulation, requiring strong turbulence or excitation to initiate full contraction of entire colony, usually contracting only partially when touched with needle.
Zooids elongate, subconical, usually 50–75 µm (n=11) long; body widest at peristomial lip, which measures 35–45 µm (n=11) in diameter ( Figs. 1 View FIGURE 1 A, K). Small number of enlarged zooids (macrozooid?) measuring up to 90– 100 × 50–60 µm usually present on middle or distal parts of secondary stalks ( Figs. 1 View FIGURE 1 B, F, J). Body slightly constricted below peristomial lip, which lacks a secondary, circumferential infolding (compare with Fig. 2 View FIGURE 2 A); epistomial disc moderately elevated above peristomial lip ( Fig. 1 View FIGURE 1 A). Pellicular striations easily visible above × 400 magnification ( Fig. 1 View FIGURE 1 M), but surface of body appears uniformly smooth at low magnifications. Telotroch discoid, measuring 25–30µm x 50 –60 µm.
Cytoplasm of zooids packed with tiny (0.8–1.5 µm diameter), dense granules visible only at high magnification (×1000); cell colorless or slightly grayish at low magnifications. Food vacuoles and items of food within them variable in size, randomly distributed in body. Single contractile vacuole in adoral position beneath epistomial disc and near dorsal wall of infundibulum. Macronucleus C-shaped, transversely oriented, surrounding micronucleus and lower half of infundibulum ( Fig. 1 View FIGURE 1 A).
Oral infraciliature as shown in Figures 1 View FIGURE 1 H, I, N-P. Haplo- and polykinety making one and one-quarter circuits around peristome and one additional circuit within infundibulum. Haplokinety and polykinety parallel on peristome, diverging within infundibulum to lie on opposite walls ( Fig. 1 View FIGURE 1 H). Epistomial membrane short, located at entrance into infundibulum (arrows in Figs. 1 View FIGURE 1 H, P). Germinal kinety running parallel to haplokinety in adoral half of infundibulum ( Figs. 1 View FIGURE 1 H, O, arrow). Each of three infundibular polykineties (P1–P3) consisting of three rows of kinetosomes. All rows of Pl terminating adstomally at level of cytostome; rows of P2 terminating adstomally at adstomal curvature of P1. Rows of P2 terminating abstomally without merging with P1; abstomal 1/4 of row 3 of P2 diverging from other rows of P2 ( Figs. 1 View FIGURE 1 H, I, N). All rows of P3 terminating adstomally at point slightly beyond adstomal end of P2 and abstomally at point approximately 1/3 of distance from adstomal to abstomal end of P2. Rows 2 and 3 of P3 closely parallel to row 1 in adstomal half, diverging from it in abstomal half, converging again with it at abstomal end of P3 ( Fig. 1 View FIGURE 1 I).
Trochal band consisting of band of dikinetids encircling cell at point 3/4 of distance from peristome to scopula.
Silverline system consisting of closely spaced, parallel, transverse silverlines ( Figs. 1 View FIGURE 1 G, Q); 50–60 silverlines present between peristome and trochal band, 22–27 between trochal band and scopula. Pellicular pores sparsely distributed alongside silverlines ( Fig. 1 View FIGURE 1 Q)
Remarks. The Chinese population of Z. plumula that we examined is characterized mainly by the following characters: alternately branched stalk, feather-shaped outline of colony, presence of larger zooids at some points on secondary stalks, and marine habitat. Characteristics of the colonies that we observed matched well with both the original description by Kahl (1933, 1935; Figs. 1 View FIGURE 1 B, C) and the redescription by Song et al. (2002; Figs. 1 View FIGURE 1 A, G). Consequently, the identification of our samples as Z. plumula is beyond reasonable doubt.
In the experience of the senior author, Z. plumula is usually abundant in eutrophic waters and is accompanied by Z. alrasheidi Ji et al., 2009 , which forms a large, leaf-shaped colony with a similar branching pattern. However, the latter species can be distinguished from Z. plumula easily by differences in infraciliature and number of silver lines, its larger zooids (80–120 × 50–60 µm vs. 50–75 × 35–45 µm), and its thick peristomial lip, which has a prominent circumferential infolding (Ji et al. 2009).
At first glance, two other marine congeners, Z. alternans Claparède and Lachmann, 1859 and Z. niveum Ehrenberg, 1838 , are very similar to Z. plumula in shape of the colony, branching pattern, and presence of macrozooids. However, zooids of both Z. alternans and Z. niveum have different sizes than those of Z. plumula (40–56 × 26–32 µm and 54–66 × 16–22 µm vs. 50–75 × 35–45 µm), and the macrozooids of both species are located on the primary stalk rather than in on the middle or distal parts of secondary branches, as in Z. plumula . In addition, Z. niveum forms a considerably larger colony (> 1 cm vs. 3 mm in Z. plumula ) ( Bauer-Nebelsick et al. 1996; Ji et al. 2006 b).
Characters Min Max Mean SD n
Number of silverlines from Z. plumula 50 60 54.5 3.36 11 peristomial lip to trochal Z. nii 47 58 52.1 3.38 15 band Z. wangi 70 85 76.4 4.63 8 P. bidulphiae 25 31 28 2.26 10 P. m a r i n a 25 30 27.5 - 4 Number of silverlines from Z. plumula 22 27 25.3 1.57 10 scopula to trochal band Z. nii 22 30 27.4 3.04 11 Z. wangi 38 50 44.2 4.46 12 P. bidulphiae 9 13 11.6 1.30 8 P. m a r i n a 10 15 12 1.79 6
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |