Cremastra saprophytica Suetsugu, 2021
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.527.2.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03E4AC2B-FF97-BC72-F7E5-25DAFEBA7AB2 |
|
treatment provided by |
Plazi |
|
scientific name |
Cremastra saprophytica Suetsugu |
| status |
sp. nov. |
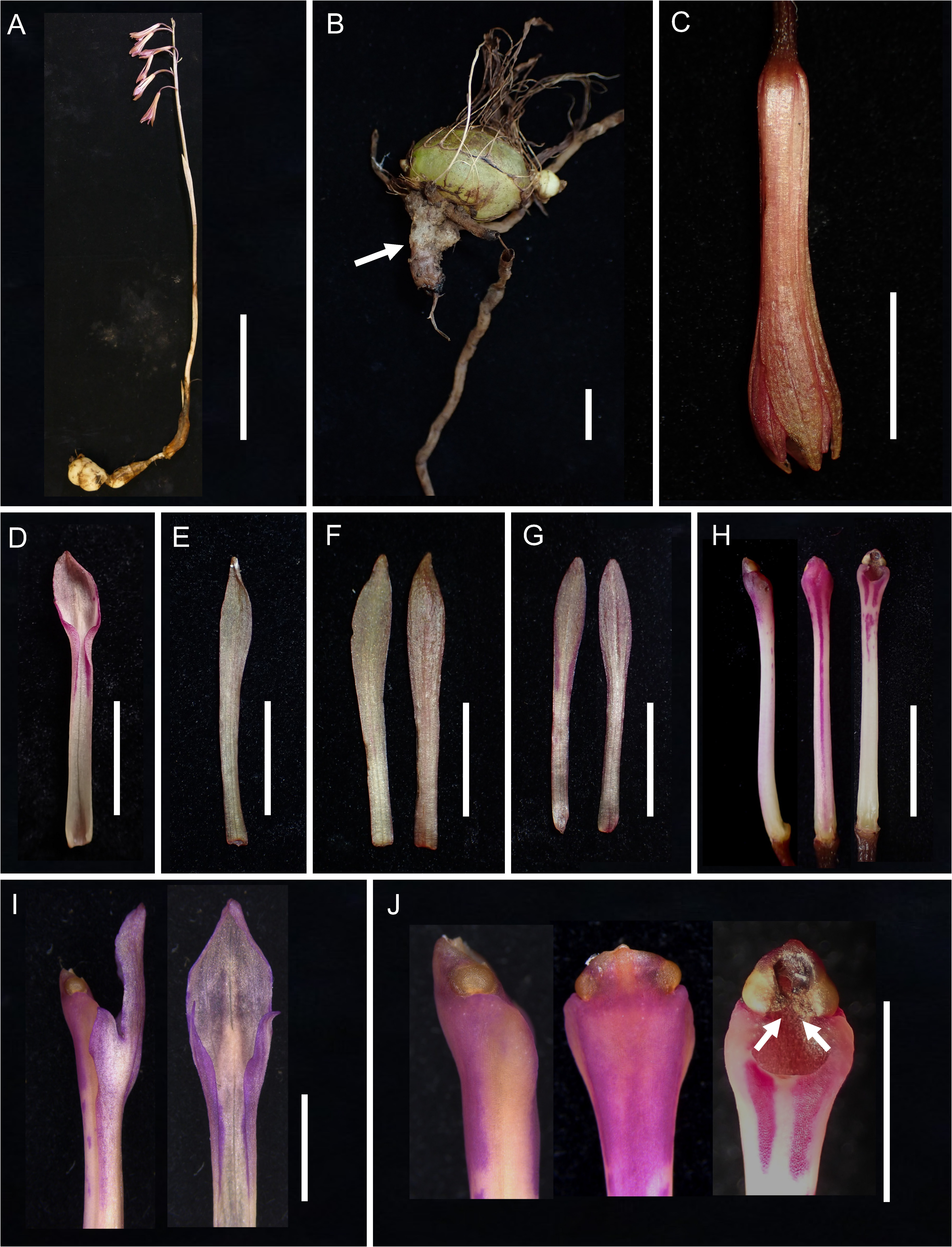
Cremastra saprophytica Suetsugu View in CoL , sp. nov. ( Figs 1 View FIGURE 1 , 2 View FIGURE 2 )
Type:— JAPAN. Gifu Pref.: Ibi County, Ibigawa Town , Kasugakawai , 5 Jun 2021, Suetsugu Sa 52 (holotype: KYO!, spirit collection) .
Cremastra saprophytica is similar to C. aphylla but differs by its more closed perianth tube, smaller lateral lip lobes, smaller callus positioned at base of the midlobe and lack of rostellum and viscidium.
Terrestrial, leafless herbs, 28–48 cm tall with subterranean tuberous pseudobulbs, creeping rhizomes and sometimes coralloid mycorrhizal rhizomes. Roots fibrous, whitish, densely hairy, spreading from the base of the pseudobulb. Inflorescence erect from the upper part of pseudobulb, 25–45 cm long, slightly purplish green with 2–3 nodes, each node with a tubular sheathing scale, the sheath 2.5–6.0 cm long, rachis 7–15 cm long, 5–22-flowered, secund, floral bracts narrowly elliptic to lanceolate, obtuse, 0.5–1.0 cm long, green. Pedicel and ovary ca. 2 times longer than floral bract, up to 24 mm, dark purple. Flowers pendulous, hardly opening, rose-purple to orange-brown, narrowly campanulate. Dorsal sepal oblanceolate-spathulate, 26.0–30.0 × 3.1–3.9 mm at the widest part, apex acute to acuminate. Lateral sepals oblanceolate-spathulate, slightly oblique, 26.0–30.0 × 3.1–3.9 mm at the widest part, apex acute to acuminate. Petals oblanceolate-spathulate 24.5–27.0 × 2.7–3.2 mm at the widest part, apex obtuse. Lip 26.0– 30.0 mm long, divided into epichile and hypochile, hypochile linear, shallowly saccate at base involute and furrowed, 18–21 mm lon, epichile trilobed from base, lateral lobes extending from inrolled margins of hypochile, 2.5–3.5 mm long, apex never exceeding column, midlobe ovate to narrowly oblong, 8.0–9.0 mm long, apex acute or obtuse; base of epichile with a smooth callus, 2.0–3.0 mm long, 0.5–1.0 mm in diameter. Column straight, 24.0– 26.5 mm long, without narrow ventral wings below anther, dilated at apex, light purple, purple surrounding stigma cavity and along midrib on ventral surface, stigma orbicular, rostellum absent, anther cap joined with column, triangular, yellow, apex thickened, pollinia 4, yellow, compressed, in 2 groups, viscidium absent. Capsules pendent, ellipsoid-cylindric, 30–33 mm long.
These measurements above are based on several specimens from the type locality and may not entirely represent the diversity present in the species when more specimens are found in the future.
Additional specimens examined:— JAPAN. Gifu Pref.: Ibi County, Ibigawa Town, Kasugakawai , 18 Dec 2020, Nishida 20201218 ( Lake Biwa Museum !)
Distribution and phenology:— Cremastra saprophytica is only known from the type locality. Flowering occurred from late May to early June, and fruiting from late June to early October.
Taxonomic notes:— Cremastra saprophytica is the second completely leafless species in the genus. It is superficially similar to C. aphylla due to their leafless and mycoheterotrophic habit ( Yukawa 1999). However, C. saprophytica can be easily distinguished from C. aphylla by its green stem, more closed perianth tube, smaller lateral lobes of lip, smaller callus of lip positioned at base of the midlobe, and absence of a rostellum and viscidium ( Figs 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 and Table 1).
In Cremastra , except in the two leafless species, the leaf emerges in the autumn and persists through the winter, withering at flowering ( Freudenstein et al. 2017). Therefore, because leafy Cremastra species often lack leaves when flowering, C. saprophytica may be confused with two varieties of C. appendiculata with relatively similar floral morphology. However, apart from its leafless habit, C. saprophytica can be distinguished from C. appendiculata var. appendiculata by the floral morphological characters mentioned above because C. aphylla and C. appendiculata var. appendiculata have identical floral structures ( Lund 1988, Yukawa 1999). In addition, although C. saprophytica is somewhat similar to C. appendiculata var. variabilis in having a small smooth lip callus on the base of midlobe ( Lund 1988), C. saprophytica can be distinguished by the other floral morphological characters mentioned above as well as its column wing condition (absent vs. a narrow ventral wing below anther).
Reproductive notes:— Cremastra saprophytica possesses an effective self-pollination system, and the fruit set is nearly 100% ( Fig. 1G View FIGURE 1 ). In other Cremastra species , a well-developed rostellum/viscidium acts as a barrier to selfpollination ( Fig. 3E View FIGURE 3 ; Chung & Chung 2003). In contrast, there is no rostellum and viscidium in C. saprophytica , resulting in contact between the pollinia and stigma ( Fig. 2J View FIGURE 2 ) and allowing autonomous self-pollination. In addition, given that the viscidium acts as a glue to bind the pollinia to insect visitors, its lack must decrease the likelihood of insect pollination. Therefore, autogamy is likely the dominant, if not exclusive, reproductive strategy in C. saprophytica . As mentioned above, C. saprophytica can be distinguished from C. aphylla by not only column morphology but also smaller flower size and more closed perianth tube ( Fig. 1 View FIGURE 1 , 3 View FIGURE 3 ) probably associated with its autonomous self-pollination system.
Self-pollination is thought to be an adaptive response that provides reproductive assurance under conditions of pollinator limitation ( Suetsugu 2013b), which is reported to be widespread among orchids ( Tremblay et al. 2004). The fruit set of entomophilous Cremastra species is far lower than average in Orchidaceae (ca. 30%; Tremblay et al. 2004), with C. appendiculata in a Korean population exhibiting only 1.3–2.0% fruit set under natural conditions, even though artificial self- and cross-pollination both resulted in nearly 100% fruit set ( Chung & Chung 2003). Therefore, it is likely that pollinator limitation severely affects the reproductive success of Cremastra species. It is also noteworthy that autonomous self-pollination has been suggested to be favourable for mycoheterotrophic plants because they are restricted to dark shaded forest understory with few pollinators ( Leake 1994, Zhou et al. 2012, Suetsugu 2013a, 2015).
Ecological notes and etymology:— Mycoheterotrophic plants have often been misrepresented by many botanists as a form of saprophytism ( Leake 1994). However, despite their leafless nature, they do not directly obtain carbon from decaying organic matter. Instead, most mycoheterotrophic plants depend on the photosynthate of adjacent autotrophic plants through shared mycorrhizal networks ( Martos et al. 2009, Suetsugu et al. 2020). However, recent studies have shown that several mycoheterotrophic orchids obtain carbon from dead wood via saprotrophic fungi ( Martos et al. 2009, Suetsugu et al. 2020). Therefore, even though the term “mycoheterotroph” has replaced the formerly misapplied term “saprophyte”, some mycoheterotrophic plants are indirectly saprotrophic ( Martos et al. 2009). The genus Cremastra is one of such examples exploiting wood-decaying Psathyrellaceae ( Suetsugu et al. 2021) . Cremastra saprophytica is also associated with Psathyrellaceae fungi, and the fruiting bodies of Coprinellus disseminates , one of the mycobionts, could be observed on decayed fallen trees near C. saprophytica plants ( Fig. 1H View FIGURE 1 ). The new species is named after its indirectly saprophytic habit.
However, it should be noted that C. saprophytica accumulates more chlorophyll than C. aphylla in the shoot ( Fig. 1A–C and 1G View FIGURE 1 ), although it is arguably at a late stage in the evolutionary development toward complete mycoheterotrophy due to its leafless habit. Given that recent studies have shown that the stems of these leafless orchids have been shown to provide some photosynthetic carbon to the plants ( Zimmer et al. 2008, Suetsugu et al. 2018, Kobayashi et al. 2021), it is likely that C. saprophytica is a partially mycoheterotrophic species rather than fully mycoheterotrophic. In particular, because the green colour deepens during fruit maturation ( Fig. 1G View FIGURE 1 ), its photosynthetic ability may significantly contribute to fruit and seed production. Notably, the underground parts of C. aphylla always consisted of pseudobulbs, roots and coralloid mycorhizomes, whereas not all C. saprophytica plants formed coralloid mycorhizomes. Because the main area of mycorrhizal colonization is coralloid rhizomes in Cremastra ( Yagame et al. 2013, Suetsugu et al. 2021), occasional lack of coralloid rhizomes might reflect some autotrophic carbon gain. Thus, comparative studies in C. saprophytica , C. aphylla and C. appendiculata could be an ideal model to understand how the photosynthetic apparatus functions in chlorophyllous but highly mycoheterotrophic orchids.
Preliminary conservation status: — Cremastra saprophytica is currently known only from a single population. The population comprises roughly ten mature plants, and at present we are not aware of any other locality where this species persists.
| KYO |
Kyoto University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.