Paracornulum fistulosum, Sutcliffe, Patricia R., Hooper, John N. A. & Pitcher, Roland, 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.197932 |
|
DOI |
https://doi.org/10.5281/zenodo.6198497 |
|
persistent identifier |
https://treatment.plazi.org/id/03E487C4-0565-D453-FF33-FE1EFAADFA7B |
|
treatment provided by |
Plazi |
|
scientific name |
Paracornulum fistulosum |
| status |
sp. nov. |
Paracornulum fistulosum View in CoL sp. nov.
( Figure 12 View FIGURE 12 , Tables 5 View TABLE 5 , 6 View TABLE 6 )
Material examined. Holotype: QMG329109 (SBD504571), seabed near Arlington Reef, Cairns, 16° 42΄ 17ʺ S 146° 0 7΄ 30 E, 47m depth, epibenthic sled, 30 ix. 2003, coll. RV Lady Basten.
Paratypes: QMG329280 (SBD537201), seabed between mainland and Gould Reef (No. 1), 19° 37΄ 30ʺ S 148° 0 5΄ 0 5 E, 45 m depth, epibenthic sled, 28 xi. 2005, coll. RV Lady Basten. QMG329191 (SBD518733), seabed near Noddy Reef No. 3, Cape York, 13° 37΄ 30ʺ S 143° 53΄ 42 E, 31m depth, epibenthic sled, 8 ii. 2005, coll. RV Lady Basten.
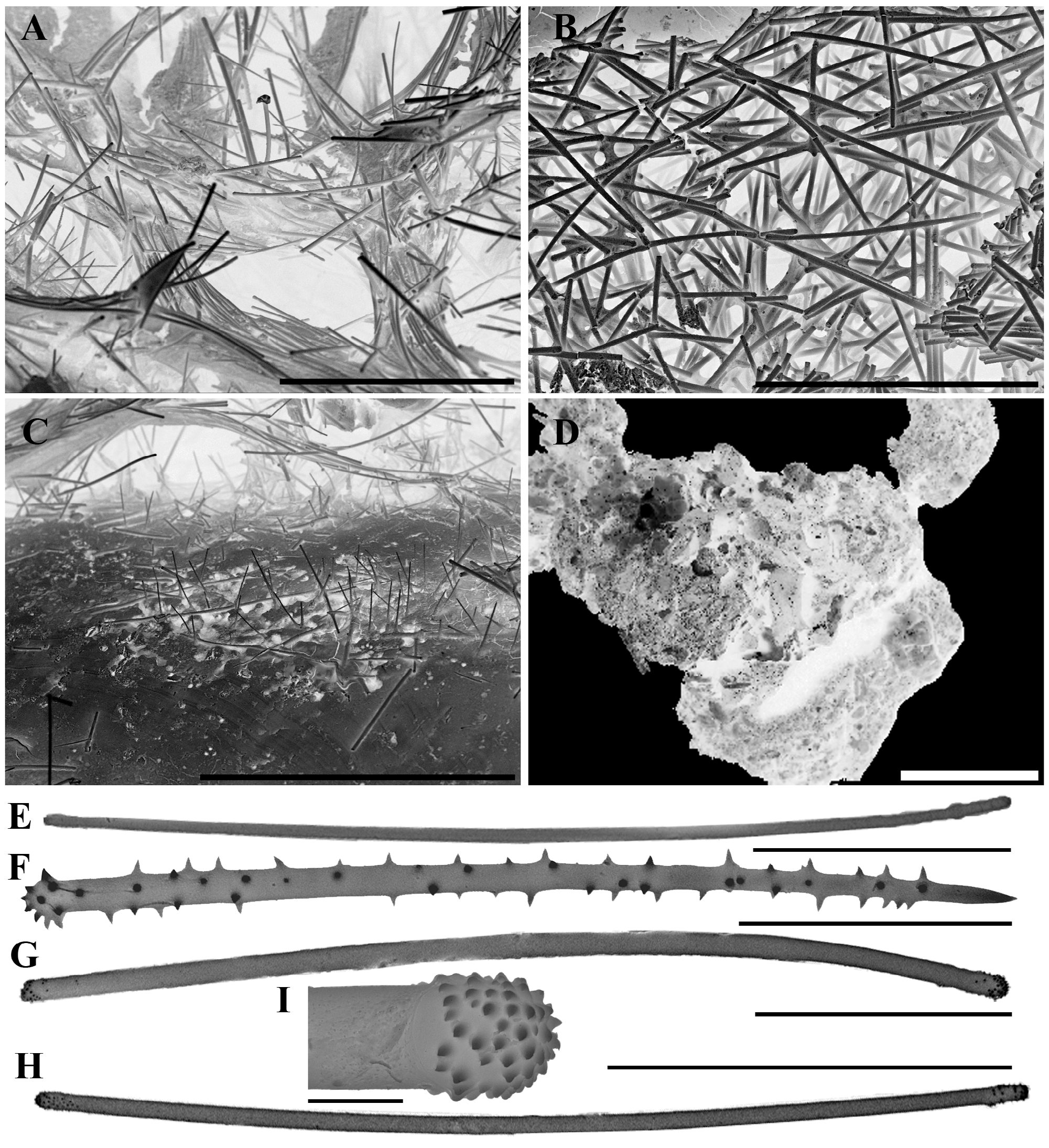
Description. Shape. Small, elongated globules from 5 to 15 cm in length observed. Thin papery fistules rise to a distance of approximately 1–2 cm from the body surface.
Colour. Dark brown detachable ectosome, uniform in colour. Main body of sponge is also brown, some specimens with a slightly yellow interior, oxidising to brown.
Oscules. Papery fistules are erect on the upper surfaces of globules. Fistules are open with a terminal oscule approximately 3 mm in diameter, composed of a thin wall less than 1 mm thick. Fistules collapse when preserved, but still recognised in most specimens, flattened along the surface of the sponge.
Texture and surface characteristics. Surface covered with a paper-like detachable crust of tangential tylotes. Specimens are brittle and easily broken due to the dominance of sand within the choanosome.
Skeletal structure. Given its small, agglutinating growth form the skeletal structure can only be determined accurately using untreated or barely treated histological preparations under scanning electron microscopy. The basal skeletal architecture is hymedesmioid with acanthostyles erect and embedded in a basal layer of spongin coating the biogenic coralline substrate. These acanthostyles appear to be patchy, as observed under SEM (presumably related to the distribution of basal spongin) with acanthostyles barely present in some places, but forming dense clumps in other places, such as areas where bioerosion of the calcite appears to be occurring (see Figure 12 View FIGURE 12 C showing a worm tube with pitted surface and paratangential acanthostyles conforming with these cavities). Arising from this hymedesmioid basal skeleton are dense tracts of tylotes forming the majority of the choanosomal skeleton, also with some thinner tylotes (Type I) incorporated. Spicule tracts loosely ascend to the surface, with a less well-developed reticulate component. Under SEM many tracts appear to be paratangential (e.g. Figure 12 View FIGURE 12 A), but this is possibly due to compression of the choanosomal skeleton during preservation (e.g. collapse of the fistules), and chemical preparation for histology. The ectosomal skeleton is a dense feltwork of tangentially arranged, apically spined tylotes forming a thin layer, clearly distinct from the choanosomal skeleton ( Figure 12 View FIGURE 12 B), producing the parchment-like ectosomal peel. Both choanosomal and ectosomal skeletons are highly spiculose with a poorer spongin component.
Megascleres ( Table 5 View TABLE 5 ). Apically spined tylotes (Type II) are the primary spicules of this species, found in both the ectosomal and choanosomal skeletons. Terminal and subterminal spination is variable, ranging from large, irregular spines concentrated in the tyle region, to finer, more blunt spines extending partially down the shaft ( Figure 12 View FIGURE 12 G, H). Smooth tylotes (Type I) are also incorporated within the choanosomal skeleton, are much thinner than the Type II tylotes and differ only from strongyles by the presence of slight terminal swelling ( Figure 12 View FIGURE 12 E).
Acanthostyles are heavily spined at the head, with irregularly spaced but nonetheless continuous spines along the length of the shaft. Tips of the acanthostyles may be spiny or smooth, usually mirroring the degree of spination on the shaft.
Microscleres. Absent.
Habitat and distribution. This species was collected from depths greater than 30 m in the central and northern Great Barrier Reef.
Etymology. Named for the fistules which are characteristic and protrude from the upper surface of the sponge.
Remarks. Allocation of this species within the family Acarnidae remains problematic, showing similarities to three genera
Cornulum Carter, 1876 View in CoL (type species Cornulum textile Carter, 1876 View in CoL ) has a plumo-reticulate skeletal structure slightly similar to the new species, but lacks acanthostyles and has palmate isochelae. The genus was recently redefined to exclude Cornulotrocha (type species Cornulotrocha cheliradians Topsent, 1927 ) by Hajdu et al. (2006). Hooper (2002) had allocated Cornulotrocha as a synonym of Cornulum View in CoL , based on similarities in skeletal structure and also on the original published description whereby megascleres were described as diactinal. Hajdu et al., (2006) subsequently found that these megascleres were actually monactinal, and together with possession of acanthostyles reallocated it to Microcionidae View in CoL as Clathria (Cornulotrocha) View in CoL . In the present species the tylotes are clearly diactinal, and together with the presence of acanthostyles, the species does not fit well with Cornulum View in CoL .
Zyzzya View in CoL is excavating, with a choanosomal skeletal structure consisting of irregular or plumose and widely spaced tracts of tylotes. Palmate isochelae may be present or absent (absent in the holotype of the type species, Z. fuliginosa View in CoL , but otherwise present in other populations; Hooper, 2002: 431), interpreted as a secondary loss, common amongst other poecilosclerids (e.g. Hooper, 1996). Zyzzya View in CoL has a strong apomorphy in the form of characteristically verticillate-spined acanthostrongyles that form a prominent secondary isodictyal reticulate skeleton. These acanthostrongyles are quite different to the category of echinating acanthostyles in the new species described here, forming the hymedesmioid basal skeleton. Van Soest et al. (1994) reported on a Fiji specimen allocated to Z. fuliginosa View in CoL that had rare acanthostyles and no proper verticillated acanthostrongyles, with its allocation to Z. fuliginosa View in CoL confirmed by the common possession of makaluvamines, a pyridoacridine alkaloid class of compound typical of this species. Consequently we checked the chemical profile of our new species, which was found not to contain makaluvamines (Mary Kay Harper & Chris Ireland, pers.comm.). Thus, its potential allocation to Zyzzya View in CoL is tenuous at best, lacking the primary Zyzzya View in CoL apomorphy of verticillate-spined acanthostrongyles in isodictyal reticulation but having an unequivocal basal hymedesmioid skeleton of echinating acanthostyles.
This new species appears to fit best with Paracornulum View in CoL on the basis that amongst Acarnidae View in CoL it possesses true acanthostyles and diactinal spicules. The new species is similar in this respect to the type species, P. dubium, ( Hentschel, 1912) View in CoL , with acanthostyles echinating a basal spongin skeleton, but differs from all other Paracornulum View in CoL in lacking microscleres (interpreted as a secondary loss), and having a choanosomal skeleton that is plumose but compressed and therefore plumo-reticulate rather than radial. A comparison of spicule composition and sizes between this new and the known species of Paracornulum View in CoL is provided in Table 6 View TABLE 6 .
Initial collections of this species from the GBR seabed included two other, cryptically similar species within a single OTU, which subsequent more detailed taxonomy revealed as a heterogeneous species complex. Although extremely similar in external morphology, the spicule components and skeletal structures were found to be clearly different. We have therefore not included associated widespread GBR distribution and biophysical data for P. fistulosum View in CoL until all of the many hundreds of specimens originally included within this OTU have been examined and assigned to an appropriate taxon.
Aside from the intrinsic value of discovering two new species amongst the five most common sponges on the GBR, the most significant contribution this study has made is to tell us how much we still do not know about the now relatively well-explored GBR. It is clear that our previous understanding of sponge biodiversity within the GBR is predominantly based on the reefal faunas, which comprise only about 7% of the GBRWHA. These five most common sponges exhibit widespread distributions and are predominantly located in sand or calcium carbonate dominated benthic habitats, as opposed to muddy, patch reef or rocky outcrop benthic habitats.
The presence of species such as D. xanthus that incorporate detritus into the skeleton, becoming a major structural component of the sponge, provides stabilisation to the benthos as it spreads through the sediment, agglutinating the biogenic rubble and inorganic substrata. This has an important follow-on effect of providing a new habitat for other species to subsequently colonise. By its very fragile nature and relatively shallow depth this community is at significant risk through both anthropogenic and naturally caused impacts. The potential loss of these habitat stabilising species through activities such as trawling, will have compounding effects on other species, with a conservation management implication. Thus, the GBR Seabed Biodiversity Project has collected data crucial to the conservation and management strategies of the GBRWHA.
Of the several environmental covariates recorded in this study, depth was found to have little relative correlation with species distributions. Conversely, substrate composition was repeatedly a highly influential factor in determining the presence of these species. For example it was mostly the presence of mud, sand or carbonate that correlated positively or negatively with particular species distributions. This contrasts with a previous study ( Cleary et al., 2005) of the benthos of the Spermonde Archipelago, Indonesia, which concluded that coral reef associated sponges had differing community structures between inshore and offshore reefs, with depth being the most important physical characteristic defining variation in diversity. However, similar to the seabed fauna of the GBR, the foraminifera, which can live in sandy, inter reef seabed habitats, were broadly distributed across the shelf and were more affected by exposure and habitat. Thus, our prior generalisations concerning trends in the distributions of sponges in coral reef ecosystems, and the environmental and physical parameters that influence these distributions, are incomplete and therefore not entirely correct.
This prior knowledge of sponge distributions shows they are highly spatially heterogenous (e.g. see summary in Hooper & Ekins, 2004). While this spatial variation cannot be completely explained by environmental variability, general influences include substrate type (e.g. Duckworth et al. 2008), reproductive methods and dispersal capabilities (e.g. Uriz et al. 1998), depth and distance from shore, where factors such as light intensity, turbidity, nutrient and sediment content are influential (e.g. Wilkinson & Cheshire 1989; de Voogt et al. 2006). Neighbouring coral reef systems have been found to have up to 85% dissimilarity in species composition, with relatively few species displaying widespread distributions. The most highly correlated factor determining the distribution of widespread species was the presence/absence of niche habitats, such as caves, reef flats, spurs and grooves or particular inter reef regions (eg. Hooper, 1994; Hooper et al., 1999).
Despite the significance of numerous environmental variables on the distribution of sponges at smaller scales, analysis of sponge diversity of the tropical Australian fauna at the continental scale found the tropical east coast to constitute a single province, with the Great Barrier Reef showing transitional zones only at Mackay/Townsville and in the far Northern region ( Hooper & Ekins 2004). This has been supported by genetic differences found within populations of the widespread species Leucetta chagosensis View in CoL , which showed significant differences between haplotypes at a line between the Whitsunday Islands and the Swain Reefs ( Wörheide et al. 2002). Most of these conclusions are based on coral reef species data.
While patch reefs and rocky outcrops are interspersed throughout the continental shelf of the GBR, they are disjointed and often isolated, sometimes with little or haphazard connectivity between them as seen previously for local sponge populations (e.g. Hooper, 1994). In contrast, seabed habitats have a far higher level of continuity over large distances, where there are no well-defined physical barriers. In the GBRSBD study predicted distributions of these species are based on analyses of the most influential physical parameters thought to affect the known distributions of seabed populations. These maps show that with the exception of D. xanthus , the predicted presence and biomass of these species are largely continuous along a latitudinal gradient. This suggests that continuous areas of similar seabed habitats provide corridors for widespread species distribution, at least for those species that are adapted to live in these inter-reef habitats.
Based predominantly on the previously known coral reef associated faunas, it was shown that the Great Barrier Reef could be divided into regions based on species distributions and species ‘turnover points’ (betadiversity) ( Hooper & Ekins, 2004), with a latitudinal turnover of the sponge fauna at the Townsville/Mackay region and in the far north of the GBR. Few coral reef associated species exhibited widespread distributions, with less than 20 reef species found in more than 12 locations (0.1%). In significant contrast to the reefassociated faunas, the inter-reef seabed species’ distributions occupy genetically connected corridors along the entire length and breadth of the GBRWHA, not previously known for any of the GBR sponges. These combined reef and inter-reef datasets represent a valuable and globally unique future research resource.
TABLE 5. Measurement of spicules for Paracornulum fistulosum sp. nov., as range (and mean) of length x width in μm, N = 30.
| Specimen number | Tylotes (Type I) | Tylotes (Type II) | Acanthostyles |
|---|---|---|---|
| QMG329109 (SBD504571) | 200–400 x 3–8 (300 x 5) | 300–450 x 2–6 (400 x 4) | 70–130 x 2–5 (95 x 3) |
| QMG329280 (SBD537201) | 145–400 x 4–10 (235 x 5) | 350–470 x 3–7 (400 x 5) | 90–130 x 2–5 (100 x 3) |
| QMG329191 (SBD518733) | 200–400 X 4–8 (350 X 6) | 330–400 X 3–7 (350 X 4) | 65–150 X 2–3 (88 X 3) |
TABLE 6. Comparisons in spicule composition and size ranges for species of Paracornulum Hallmann, 1920 (data from original descriptions).
| Tylotes | Acanthostyles Styles | Chelae Toxas | Microrhabds |
|---|---|---|---|
| P.dubium View in CoL (Hentschel, 216–440 μm 1912) (type species) (Two size classes) | 96–152 μm absent | 14–16 μm 80–150 μm | absent |
| P. c o h e re n s Levi, 250–300 x 10 μm 1963 | 150–275 x absent 12–14 μm | 20 μm absent | 12 μm |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Paracornulum fistulosum
| Sutcliffe, Patricia R., Hooper, John N. A. & Pitcher, Roland 2010 |
P. sinclairae
| Bergquist & Fromont 1988 |
Cornulotrocha cheliradians
| Topsent 1927 |
P. strepsichela
| Dendy 1922 |
Paracornulum
| Hallmann 1920 |
P. dubium, (
| Hentschel 1912 |
Cornulum
| Carter 1876 |
Cornulum textile
| Carter 1876 |