Ecdyonurus (Rhithrogeniella) ornatus ( Ulmer 1939 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5319.4.2 |
|
publication LSID |
lsid:zoobank.org:pub:20272586-E197-4DEA-811F-496572F121B7 |
|
DOI |
https://doi.org/10.5281/zenodo.8211535 |
|
persistent identifier |
https://treatment.plazi.org/id/03E48787-FFCE-E640-FF64-F96B8FDCFD3B |
|
treatment provided by |
Plazi |
|
scientific name |
Ecdyonurus (Rhithrogeniella) ornatus ( Ulmer 1939 ) |
| status |
|
Ecdyonurus (Rhithrogeniella) ornatus ( Ulmer 1939)
( Figs 1–102 View FIGURES 1–10 View FIGURES 11–25 View FIGURES 26–33 View FIGURES 34–41 View FIGURES 42–52 View FIGURES 53–68 View FIGURES 69–72 View FIGURES 73–76 View FIGURES 77–84 View FIGURES 85–90 View FIGURES 91–96 View FIGURES 97–102 , 110 View FIGURES 103–110 )
Rhithrogeniella ornata Ulmer 1939: 576 , figs 169–174 (♂ and ♀ imagines and subimagines); Sartori 2014: 49 View Cited Treatment (♂ and ♀ imagines and subimagines, eggs and larvae);
Rhithrogena ornata: Wang & McCafferty 2004: 21 View in CoL .
Rhithrogeniella tonkinensis Soldán & Braasch 1986: 206 (♀ imago, ♂ subimago, larva, egg) syn. n.; Boonsoong & Braasch 2013: 78 (larva, egg).
Ecdyonurus tonkinensis: Wang & McCafferty 2004: 21 View in CoL .
Ecdyonurus (Rhithrogeniella) tonkinensis: Kluge 2022: 168 View in CoL (subimago).
Lectotype designation. Lectotype (designated here): male imago in alcohol, collected by Lieftinck in Buitenzorg (currently Bogor) in July 1932 and deposited in Zoologisches Museum und Biozentrum Grindel, Hamburg, Germany. This specimen is reported in literature as the following :
1) Ulmer 1939, p. 578: « 1 ♂, 2 ♀. wohl frisch geschlüpft, in Spiritus , Buitenzorg , VII. 1932, Dr. LIEFTINCK leg. (1 ♂, 1 ♀ in meiner Sammlung, Typen)»;
2) Sartori 2014, p. 49: ́One male holotype, one female allotype: Indonesia, Java, Buitenzorg , VII 1932, Dr. Lieftinck leg. [ ZMH] »;
3) Sartori, Kubiak & Michalik 2016, p. 30: ́The holotype of Rh. ornata is a specimen stored in ethanol and originating from Indonesia, Java, Buitenzorg , [Bogor], collected in July 1932 by Dr. Lieftinck ».
Formerly ( Sartori 2014, Sartori et al. 2016) this specimen was regarded to be the holotype. However Ulmer (1939) did not designate a single specimen as the holotype and did not distinguish two specimens (the male imago and the female imago) as holotype and allotype, but reported both them as types of equal status.According to Article 73 of the International Code of Zoological Nomenclature (4th edition), ́73.1.1. If an author when establishing a new nominal species-group taxon states in the original publication that one specimen, and only one, is the holotype, or “the type”, or uses some equivalent expression, that specimen is the holotype fixed by original designation» and ́73.1.3. The holotype of a new nominal species-group taxon can only be fixed in the original publication and by the original author». Thus, according to the Article 73.2 of the Code, both specimens reported by Ulmer (1939) as ́Typen» were syntypes, i.e. name-bearing types of equal status. Here one of them is designated as the lectotype.
Material examined. INDIA: state Karnataka, border of Shivamogga and Udupi districts, near Agumbe , rivers Modi-hole and Seethanadhi-hole , 11–31.I.2013, coll. N. Kluge & L. Sheyko: 1 L-S-I ♂, 1 L-S ♂, 1 S ♂, 2 L-S-I ♀, 1 S ♀, 16 larvae ( ZIN); state Tamil Nadu, Dindigul, Kodaikanal hills, Perumal malai stream, 28–30.XII.2022, coll. P. Srinivasan & R. Isack: 1 L-S-I ♂, 2 L /S ♂, 1 mature larva ♀, 5 larvae ( AMC) .
THAILAND: Kanchanaburi province, river Kwai-Yai (= Khwae Yai = Si Sawat ) and river Taphoen , Lad-Ya (= Lat Ya ), resort «Island Resort River Kwai», 29–30.I.2015, coll. N. Kluge & L. Sheyko: 1 L-S ♂, 3 L-S-I ♀, 3 larvae ( ZIN); Mae-Hong-Son province, 90 km NW Chiang-Mai, river Pai upstream Pai, 5–14.II.2015, coll. N. Kluge & L. Sheyko: 2 L-S-I ♂, 1 L-S/I ♂, 1 S-I ♂, 1 L /S ♂, 2 L-S-I ♀, 1 L-S ♀ ( ZIN) .
Descriptions
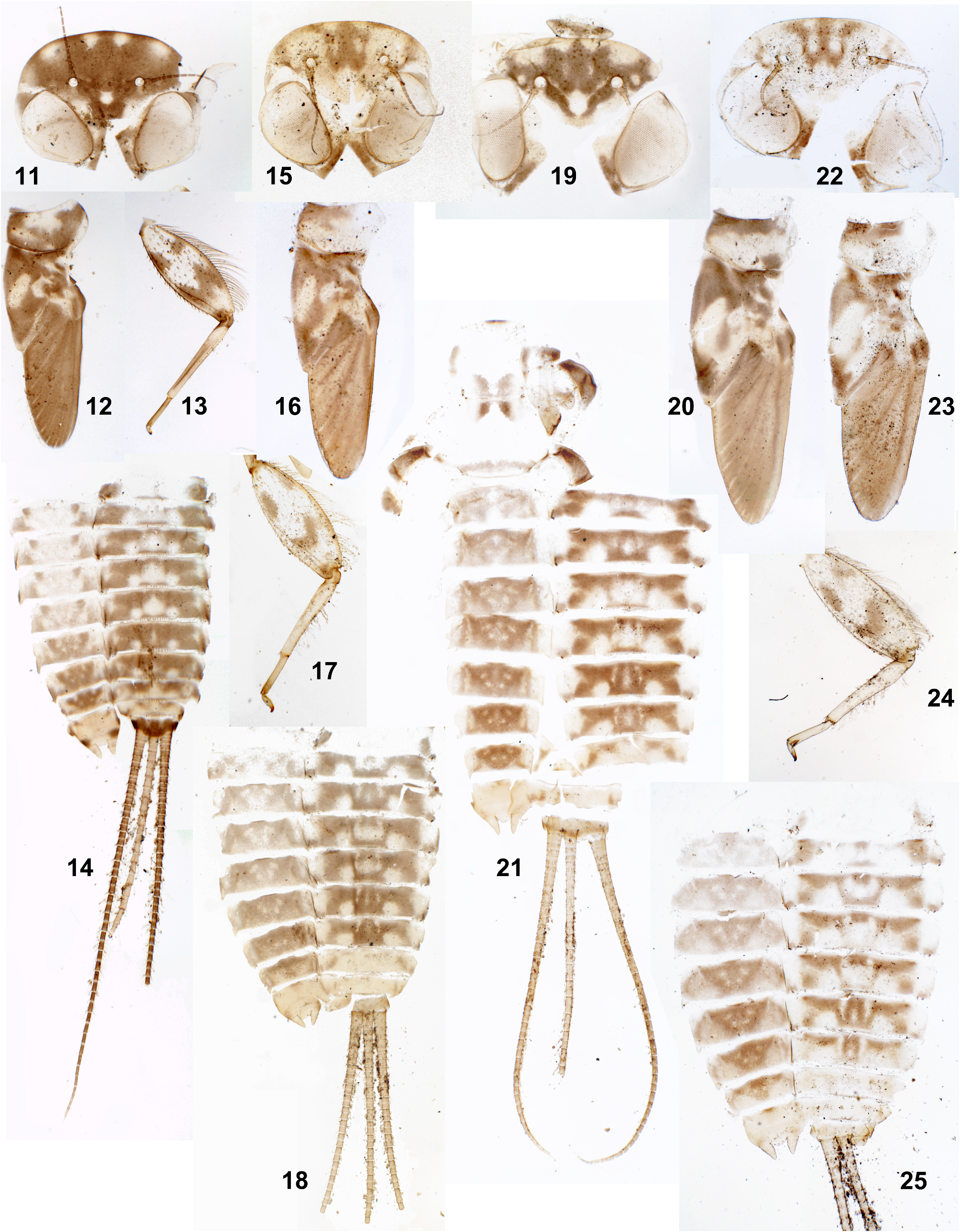
Larva. CUTICULAR COLORATION: Cuticle either pale ochre, or with more or less contrasting brown areas and ochre blanks ( Figs 11–25 View FIGURES 11–25 ). Head usually with pair of submedian blanks adjacent to anterior margin; often with more or less expressed pair of wider blanks laterad of them ( Figs 11, 15, 19, 22 View FIGURES 11–25 ; Soldán & Braasch 1986: fig. 16; Sartori 2014: fig. 6); rarely submedian blanks absent ( Sartori 2014: fig. 7). Each femur mostly light, with 3 dark maculae originating from two initial transverse bands: initial proximal transverse band is separated into two longitudinal maculae; distal transverse band integral, either V-shaped ( Fig. 17 View FIGURES 11–25 ), or Z-shaped ( Fig. 13 View FIGURES 11–25 ). Tibia mostly light, with base darkened. Abdominal terga II–VII with following blanks: median blank and pair of submedian blanks (submedian sigilla) are either separated, or fused into integral median blank; pair of smaller or larger blanks are located laterad of it and adjacent to posterior margin; pair of smaller or larger lateral blanks are adjacent to anterior margin. Terga I and VIII–IX with more extensive blanks. Abdominal sterna with small blanks corresponding to sigilla.
HYPODERMAL COLORATION: Abdominal terga with or without reddish markings resembling that of winged stages ( Figs 53 View FIGURES 53–68 ).
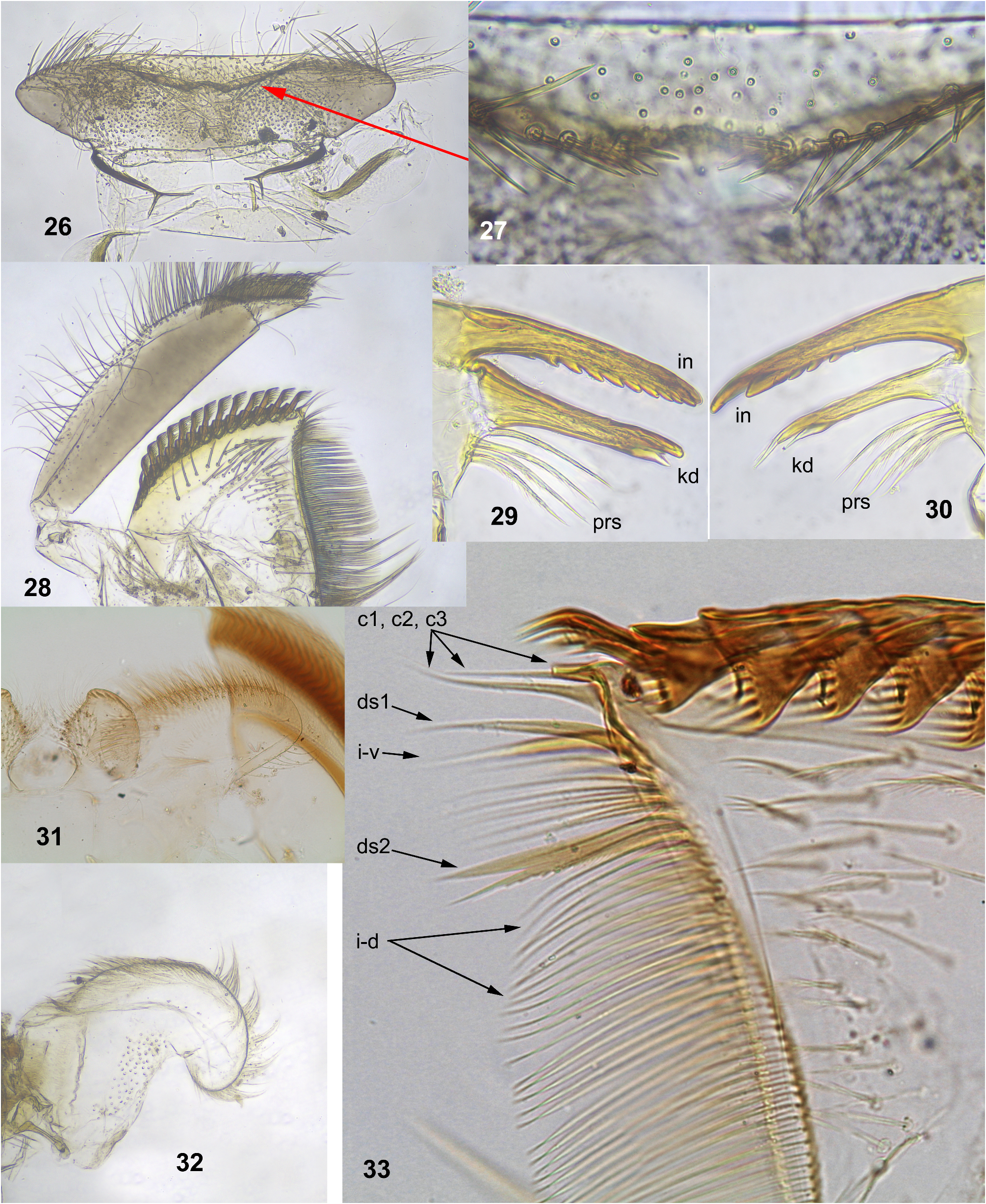
SHAPE AND SETATION: Labrum moderately expanded laterally ( Fig. 26 View FIGURES 26–33 ); anterior portion sharply bent ventrally and invisible from above, with narrow median emargination; initial anterior margin (hidden on ventral side) with pair of regular setal rows separated by median emargination; setae of these rows stout, pointed, spine-like, inclined medially toward emargination ( Fig. 27 View FIGURES 26–33 ); dorsal surface of labrum with irregular, long, hair-like setae. Mandibles as described by Soldán & Braasch (1986: 208, figs 11–12) and Sartori (2014: 52); prostheca of left mandible consists of 3–4 setae, prostheca of right mandible consists of 2–4 setae ( Figs 29–30 View FIGURES 26–33 ). Maxilla with 10–15 comb-like setae, each with 4–11 denticles ( Figs 28, 33 View FIGURES 26–33 ). Distal dentiseta simple, proximal dentiseta bifurcate, with proximal branch pectinate ( Fig. 33 View FIGURES 26–33 ; Sartori 2014: fig. 12). Hypopharynx and superlinguae usual for Ecdyonurus s. l. ( Fig. 32 View FIGURES 26–33 ; Soldán & Braasch 1986: fig. 2; Sartori 2014: fig. 11). Labium with paraglossae sharply expanded; glossae with oblique ridges on apex ( Fig. 31 View FIGURES 26–33 ; Soldán & Braasch 1986: fig. 9; Sartori 2014: figs 9–10).
Spatulate setae on dorsal surface of each femur flat, colorless, with narrow base, divergent margins and rounded apex ( Fig. 35 View FIGURES 34–41 ). Tibia and tarsus of each leg with fewer similar setae ( Fig. 40 View FIGURES 34–41 ); middle and hind tibiae with sparse row of hair-like setae on outer margin ( Fig. 39 View FIGURES 34–41 ). Claw with rectangular projection at midlength and with one row of 3–6 denticles distad of it ( Fig. 41 View FIGURES 34–41 ; Soldán & Braasch 1986: fig. 13; Sartori 2014: fig. 18)
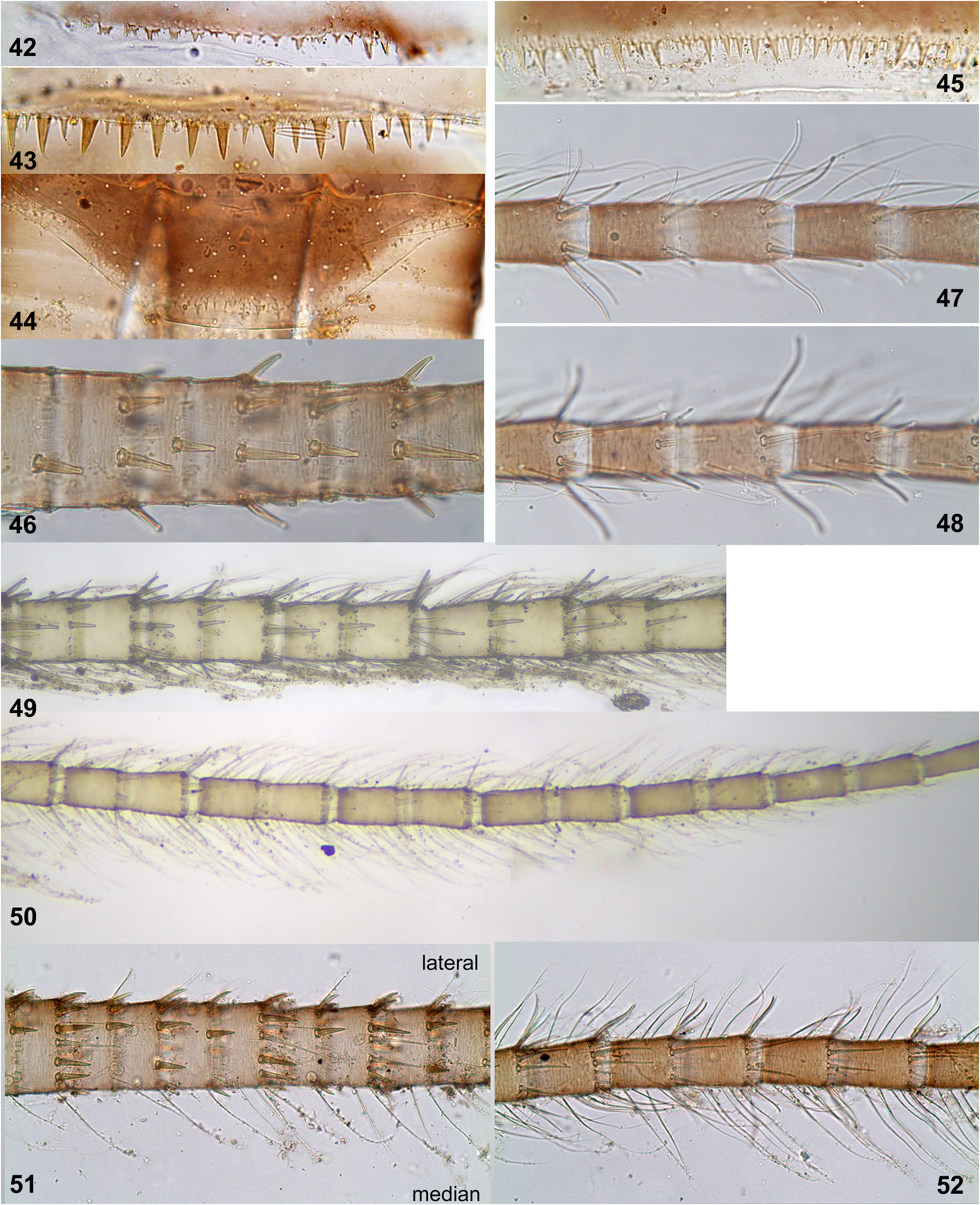
Denticles on posterior margins of abdominal terga irregular, either narrow and sharply pointed, or dentate; denticles on terga I and X very small ( Figs 42, 44 View FIGURES 42–52 ), denticles on terga II–IX larger ( Figs 43, 45 View FIGURES 42–52 ). Abdominal sterna without denticles. Tergalii increasing from II to V; tergalii I–VI with branched fibrillose portion, tergalii VII without fibrillose portion ( Figs 4–10 View FIGURES 1–10 ). Number of branches of fibrillose portion either subequal on all tergalii I–VI, or less on tergalius VI ( Fig. 9 View FIGURES 1–10 ); among individuals examined, number of branches on tergalius VI varying from 2 to 12.
Subimago. CUTICULAR COLORATION: Head colorless, antennae light brown. Pronotum colorless. Mesonotum mostly colorless, with brown anterior chromozone (area anteriad of mesonotal suture), paired brown area including antelateroparapsidal suture and paired brown parascutellar chromozone (ovoid macula on parascutellum); paired lateroparapsidal chromozone (stretching along lateroparapsidal suture and widening posteriorly) either lighter brown ( Fig. 66 View FIGURES 53–68 ), or colorless ( Fig. 67 View FIGURES 53–68 ). Thoracic pleura and sterna colorless, with ventral ark of prealar bridge narrowly bordered with light brown ( Fig. 110 View FIGURES 103–110 ). Wing membrane colorless, microtrichia brown. Legs from light brown to nearly colorless; fore leg darker than middle and hind legs. Abdominal terga and sterna nearly colorless; gonostyli light brownish; cerci proximally colorless, distally light brownish.
HYPODERMAL COLORATION: As in imago.
TEXTURE: In both sexes, on all leg pairs, all tarsomeres covered with blunt microlepides; microtrichia occupy only ventral-proximal part of first tarsomere ( Fig. 65 View FIGURES 53–68 ) ( Kluge 2022).
Imago, male ( Figs 57, 64 View FIGURES 53–68 ). Head ochre with brown. Eyes gray.
Pronotum ochre with brown macula medially (as in females — Figs 77–84 View FIGURES 77–84 ). Mesonotum with ochre and light brown areas. Thoracic pleura and sterna light ochre (as in female — Fig. 77 View FIGURES 77–84 ). Wing membrane colorless; veins mostly colorless, some longitudinal veins ochre or light brown ( Figs 57, 64 View FIGURES 53–68 ; as in Figs 58–61 View FIGURES 53–68 ). Pterostigma with simple and complete crossveins perpendicular to Sc ( Fig. 58 View FIGURES 53–68 ). Hind wing narrowing toward apex, with small, pointed costal projection ( Fig. 61 View FIGURES 53–68 ). On fore leg, femur gradually changing color from light ochre base to dark reddish-brown apex, with reddish-brown darkening at midlength; tibia ochre with base and apex contrastingly dark brown; tarsus ochre with apex brown ( Fig. 62 View FIGURES 53–68 ). Middle and hind legs lighter ( Fig. 63 View FIGURES 53–68 ).
Abdomen mostly ochre, all terga I–X with reddish-brown paired submedian stripe and ochre submedian sigilla on its background ( Figs 54–57 View FIGURES 53–68 ). Penis with proximal ⅔ unpaired, distal ¼ divided into pair of rounded lobes separated by wide space ( Figs 69–73 View FIGURES 69–72 View FIGURES 73–76 ; Sartori et al. 2016: fig. 2); latero-dorsal and ventral (discal) spines absent. Pair of median titillators present or absent; if present, with single apical point ( Fig. 73 View FIGURES 73–76 ). Cerci ochre, in proximal part with brown articulations ( Fig. 68 View FIGURES 53–68 ).
Imago, female. Coloration similar to male ( Figs 77–84 View FIGURES 77–84 ).
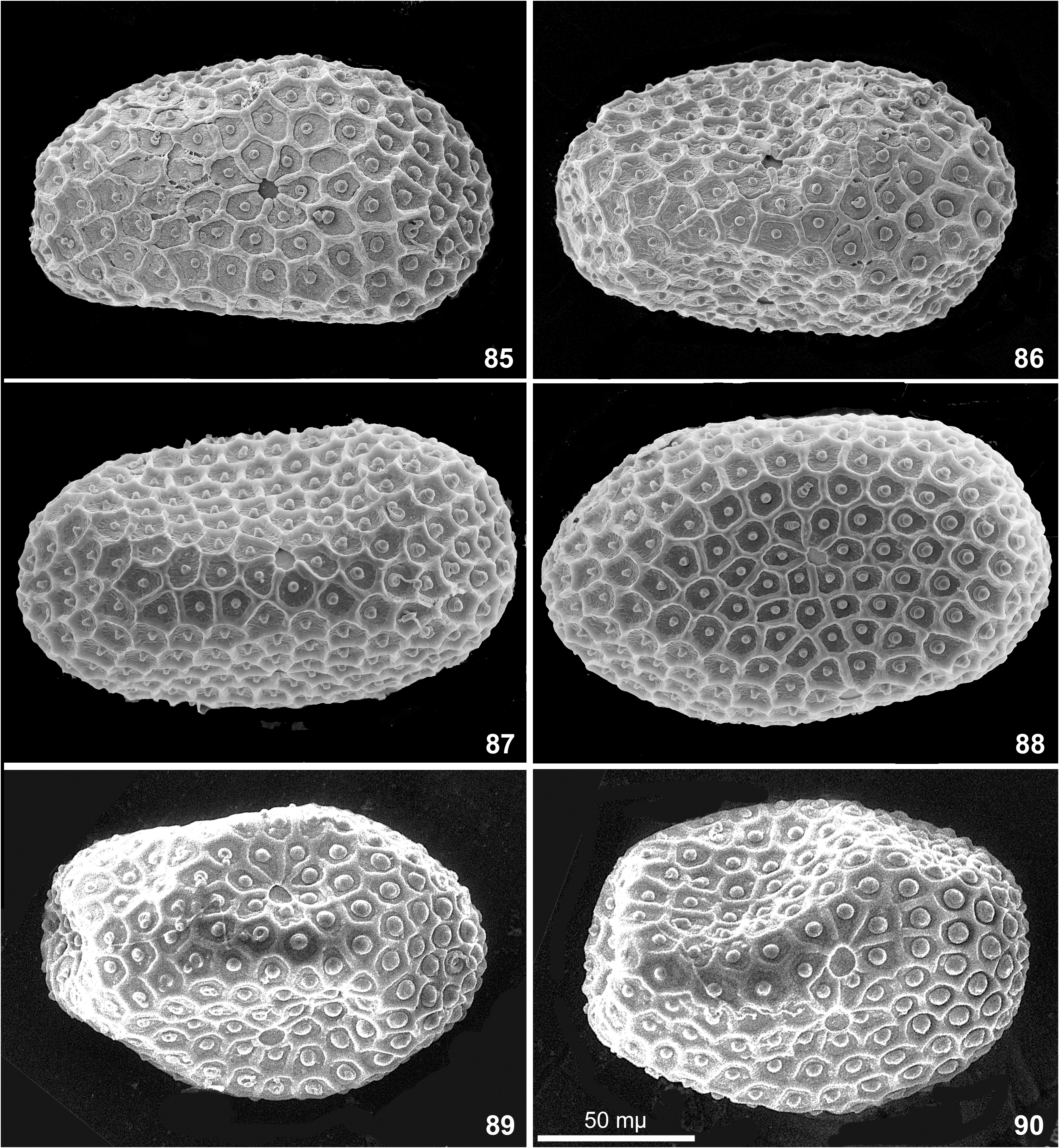
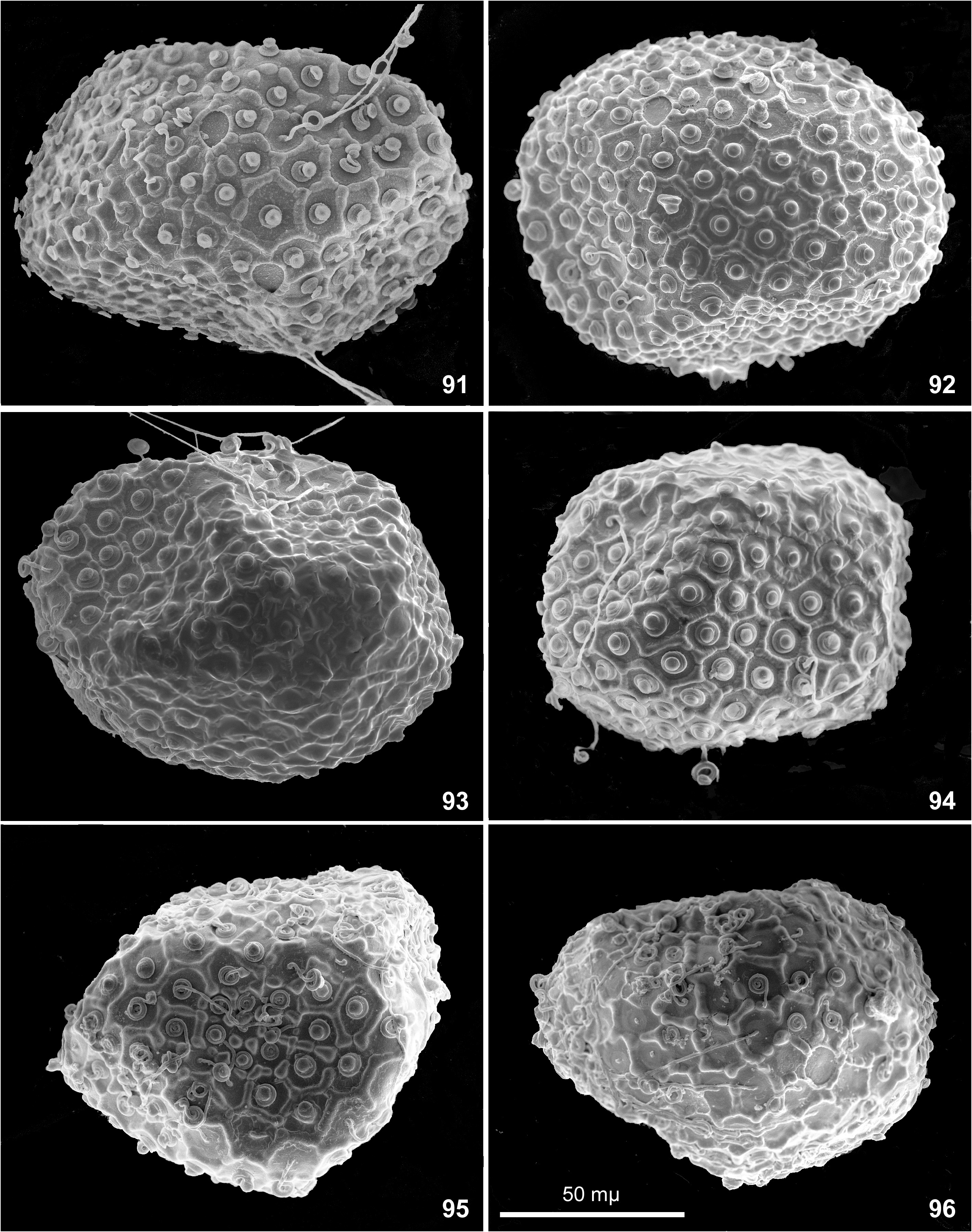
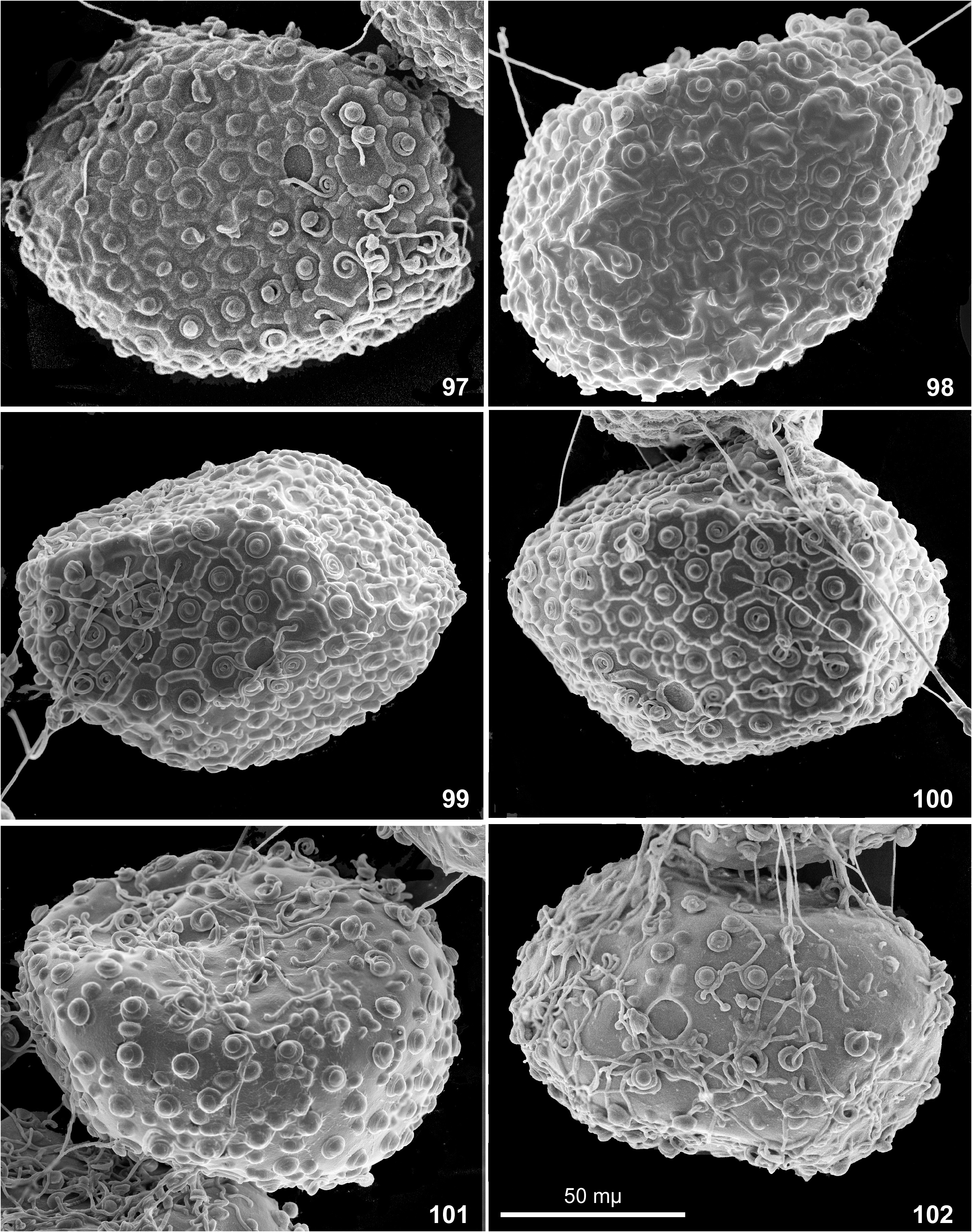
Egg. Chorion with net-like relief bearing knob-terminated coiled thread (KTC) in each cell. Among individual females, ridges forming cells vary from integral ( Figs 85–92 View FIGURES 85–90 View FIGURES 91–96 ) to interrupted ( Figs 96–97, 99–100 View FIGURES 91–96 View FIGURES 97–102 ) or irregular ( Fig. 93, 95. 98 View FIGURES 91–96 View FIGURES 97–102 ); on eggs extracted from one female completely absent ( Figs 101–102 View FIGURES 97–102 ). Among individual females, KTC varying from small ( Figs 85–87 View FIGURES 85–90 ) to large ( Fig. 91–94 View FIGURES 91–96 ) or large on one pole and small on another pole ( Figs 89–90 View FIGURES 85–90 ). Micropyle varying from small ( Figs 85–87 View FIGURES 85–90 ) to large ( Fig. 90 View FIGURES 85–90 ).
Dimension. Fore wing length 5–6 mm (up to 7 mm according to Ulmer 1939).
Distribution. Oriental Region; known from Southern India, Indochina and Great Sunda Islands.
Confusion about labrum structure. Soldán and Braasch (1986) overlooked median emargination of labrum, and Sartori (2014) erroneously reported that labrum has ́no anteromedian emargination ... ventral face with shorter and stout setae along the anterior margin». On the photo ( Sartori 2014: fig. 8), focus is given on the dorsal side, but if enlarge the picture, it is possible to see the stout pointed setae bordering the initial anterior margin, which is bent ventrally; medially this paired setal row is interrupted by the median emargination of the initial anterior margin, but this emargination is invisible on the photo; shape of this labrum and its visible setal rows are exactly the same as in our specimens ( Figs 26–27 View FIGURES 26–33 ).
Disagreements about presence / absence of titillators. Originally, male imago of Rhithrogeniella ornata was described as lacking titillators ( Ulmer 1939: 575, fig. 169); male subimagines of this species were reported, but their genital structure was not described ( Ulmer 1939: 577).
Soldán and Braasch (1986) described subimagines placed by then in a new species of Rhithrogeniella , Rh. tonkinensis , which have well developed medial titillators with sclerotized apices. In the same paper, they reported that ́similar structures occur in subimagoes of R. ornata (material in the Zoological Museum, Hamburg)» and wrote: ́Our opinion agrees with that of Jensen (pers. comm.) who supposes the existence of medial titillators as well».
Sartori (2014) and Sartori et al. (2016) reexamined the single male imago and the male subimagines which belong to the type series of Rhithrogeniella ornata and found out that the male imago has no titillators, and a male subimago has titillators which exist on subimaginal cuticle only, but are absent on imaginal penis developing under the subimaginal cuticle ( Sartori 2014: fig. 4). Based on this fact, they concluded that Rhithrogeniella (both Rh. ornata and Rh. tonkinensis ) have no titillators in imaginal stage, but have them in subimaginal stage only ( Sartori 2014: 59).
Our recent examination of the specimens from India and Thailand reveals that presence/absence of titillators in imaginal stage varies individually ( Table 1 View TABLE 1 ). All 6 examined males from India (two imagines, two subimagines and two larvae ready to molt to subimago) and one examined male subimago from Kwai-Yai in Thailand, have either imaginal titillators ( Figs 69–71 View FIGURES 69–72 ), or tissues of future imaginal titillators located under cuticle of subimaginal titillator ( Fig. 75 View FIGURES 73–76 ). Among specimens from Pai ( Thailand), 4 reared male imagines also have imaginal titillators ( Figs 72–73 View FIGURES 69–72 View FIGURES 73–76 ), but one larva ready to moult to subimago, has only subimaginal cuticle of titillators, without tissues of imaginal titillators under it ( Fig. 76 View FIGURES 73–76 ).
Thus, in all cases subimaginal cuticle bears a pair of titillators in a form of very small, pointed denticles ( Figs 74–76 View FIGURES 73–76 ); tissues of imaginal penis developing under these pointed denticles, either have a pair of larger imaginal titillators ( Fig. 75 View FIGURES 73–76 ), or lack them ( Fig. 76 View FIGURES 73–76 ); in the last case imago will have no titillators.
Variability of egg structure. Presence of mesh-like reticulate ridges was reported as diagnostic character of the subgenus Nixe ( Flowers 1980) and the genus Rhithrogeniella ( Sartori 2014) . Our recent examination of the specimens from India and Thailand reveals that presence/absence of this relief varies individually, at least among specimens from Thailand ( Table 2 View TABLE 2 ).
Geographical variability. Our small collection allows to make following assumptions about characters peculiar for certain geographical forms ( Table 3 View TABLE 3 ).
Thailand. Denticles on posterior margins of abdominal terga II–IX are irregular, small and thin ( Fig. 45 View FIGURES 42–52 ), on some of these terga can be as small as on terga I and X. In all specimens from Kwai-Yai, larval cuticle is very pale and nearly lacks pigmentation, so that the features of cuticular coloration characteristic for individuals from Pai and India, are not expressed. All examined mature larvae from Pai have well expressed cuticular coloration. Frontal shield has a pair of blanks behind the pair of submedian blanks adjacent to anterior margin ( Figs 15, 19, 22 View FIGURES 11–25 ), in contrast to the specimens from India ( Fig. 11 View FIGURES 11–25 ), Vietnam and Sumatra ( Soldán & Braasch 1986: fig. 16; Sartori 2014: figs 6–7). Cuticle of larval pronotum has dark area of this or that shape adjacent to anterior margin ( Figs 16, 20, 23 View FIGURES 11–25 ); the same in larvae from Vietnam ( Soldán & Braasch 1986: fig. 17) and Sumatra ( Sartori 2014: figs 6–7).
India. Denticles on posterior margins of abdominal terga II–IX are regularly alternating as large and small ( Fig 43 View FIGURES 42–52 ), well different from small denticles on terga I and X ( Figs 42 and 44 View FIGURES 42–52 ). All examined mature larvae from both localities in the states Karnataka and Tamil Nadu have well expressed cuticular pigmentation (in contrast to the specimens from Kway-Yai in Thailand). In contrast to the individuals from Pai in Thailand, frontal shield has no additional blanks behind the pair of submedian blanks adjacent to anterior margin ( Fig. 11 View FIGURES 11–25 ); the same is figured for the individuals from Vietnam ( Soldán & Braasch 1986: fig. 16) and from Sumatra ( Sartori 2014: figs 6–7). Cuticle of larval pronotum has paired light blank adjacent to anterior margin ( Figs 2 View FIGURES 1–10 , 12 View FIGURES 11–25 ); this differs from larvae from Pai in Thailand, Vietnam and Sumatra (see above).
In all three examined females, all eggs have regular mesh-like relief consisting of non-interrupted ridges (Figs 85–90 and Table 2 View TABLE 2 ).
Synonymy of E. (Rh.) ornatus and E. (Rh.) tonkinensis . Originally Rh. ornata was described from Great Sunda Islands (Java and Sumatra), and Rh. tonkinensis was described from Indochinese Peninsula ( Vietnam). The original differential diagnosis of Rh. tonkinensis contained the following statement: ́Since the adult male of Rhithrogeniella tonkinensis sp. n. and nymphs of R. ornata Ulmer are unknown only subimagoes of these two species can be compared» ( Soldán & Braasch 1986: 209).
Subimagines of these species were said to differ by abdominal coloration, leg proportions and shape of male genitalia.
The difference in abdominal coloration was caused by the fact that the Soldán’s drawing of subimaginal abdomen ( Soldán & Braasch 1986: fig. 19) was probably made from subimago just emerged and not fully colored; it resembles the subimaginal abdomen extracted from larva ready to molt ( Fig. 53 View FIGURES 53–68 ), while the Ulmer’s drawing ( Ulmer 1939: fig. 170) was probably made from an older subimago, whose coloration is approximated to the imaginal one ( Figs 54–57 View FIGURES 53–68 ). Female abdomen of Rh. tonkinensis was said to be ́without tergal markings», while abdomen of female Rh. ornata has tergal markings ( Ulmer 1939: fig. 171). Actually abdominal markings of female are worse expressed than in male ( Figs 78–84 View FIGURES 77–84 ). Hypodermal coloration of male abdomen, female pronotum, mesonotum and abdomen of our specimens from India and Thailand are the same as in the paralectotypes of E. (Rh.) ornatus from Java and Sumatra ( Ulmer 1939: figs 170–172).
Comparison. Beside s E. (Rh.) ornatus , three other Oriental species were described based on larvae and eggs from Taiwan, under the names Nixe (Nixe) littorosus , Nixe (Nixe) mitificus and Nixe (Nixe) obscurus ( Kang & Yang 1994) . Originally, the subgenus Nixe was characterized by absence of fibrilliform portion on tergalius VI of larva and ́mesh-like reticulate ridges» on egg ( Flowers 1980). Kang & Yang (1994) reported only the ́mesh-like reticular ridges» and ignored the structure of tergalii. It is unclear from the descriptions of these species, if they have the fibrilliform portion on tergalius VI or not. Characters reported as diagnostic for these species, vary individually in E. (Rh.) ornatus . Since imagines of these species are unknown, it is unclear if they really represent three different species distinct from E. (Rh.) ornatus , or not.
Among species examined, E. (Rh.) joernensis (widely distributed from Scandinavia and northern part of Russian Plain to Siberia, Kazakhstan, Mongolia and Russian Far East) is most closely related to E. (Rh.) ornatus . Eggs of E. (Rh.) joernensis have the same mesh-like ridges ( Flowers 1986: fig. 3), larva has the same cuticular coloration and leg setation; in both species paraglossae are greatly projected laterally ( Fig. 31 View FIGURES 26–33 ; Kluge 1997: tab. 15: fig. 7). Both species have similar individual variability of subimaginal mesonotum (compare Figs 66–67 View FIGURES 53–68 with Kluge 1980: figs 93–94). Imagines of these species have similar general appearance, including coloration of the body and legs, shape of hind wing, proportion of tarsal segments. Male imago of E. (Rh.) ornatus well differs from E. (Rh.) joernensis by more simple shape of penis and by absence of discal spines on ventral side. Larva of E. (Rh.) ornatus differs from E. (Rh.) joernensis by size of tergalii, which increase from tergalius II to tergalius V ( Figs 4–10 View FIGURES 1–10 ), while in E. (Rh.) joernensis (= E. mongolicus ) they decrease from tergalius II to tergalius VII ( Kluge 1980: fig. 84).
| ZMH |
USA, Illinois, Chicago, Field Museum of Natural History (also used by Finnish Museum of Natural History) |
| ZIN |
Russia, St. Petersburg, Russian Academy of Sciences, Zoological Institute |
| ZMH |
Zoologisches Museum Hamburg |
| ZIN |
Russian Academy of Sciences, Zoological Institute, Zoological Museum |
| R |
Departamento de Geologia, Universidad de Chile |
| AMC |
Department of Biologics Research |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Ecdyonurus (Rhithrogeniella) ornatus ( Ulmer 1939 )
| Kluge, Nikita, Sivaruban, T., Srinivasan, Pandiarajan, Barathy, S. & Isack, Rajasekaran 2023 |
Ecdyonurus (Rhithrogeniella) tonkinensis: Kluge 2022: 168
| Kluge, N. J. 2022: 168 |
Ecdyonurus tonkinensis:
| Wang, T. - Q. & McCafferty, W. P. 2004: 21 |