Cotinis aliena Woodruff, 2008
|
publication ID |
https://doi.org/ 10.5281/zenodo.5169990 |
|
persistent identifier |
https://treatment.plazi.org/id/03E387CD-9B37-877F-4CC1-FEF7F2C6FBAC |
|
treatment provided by |
Felipe |
|
scientific name |
Cotinis aliena Woodruff |
| status |
sp. nov. |
Cotinis aliena Woodruff , new species
(Fig. 1, 2, 5, 6, 9, 10, 13, 14, 17-28)
Holotype male. FLORIDA: Monroe County, Islamorada, Junction US Rt. 1 and Jerome St. , Ekblom Nursery , VII-VIII-1976, Ekblom Family. Deposited in Florida State Collection of Arthropods.
Diagnosis (habitus Fig. 1). Medium sized (L. 20.0 mm, W. 11.5 mm), typical member of the genus Cotinis . Entire dorsum and venter bright shiny, metallic green, except pygidium, which is bicolored orange and green. Elytron with 2 noticeable discal costae coalescing near posterior declivity, apically truncate, sutural apex not prolonged into a sharp tooth. Punctation not coarse nor deep, but readily visible to naked eye. Clypeal process triangular, upturned vertically; frontal process free for part of length and not extending to clypeal process.
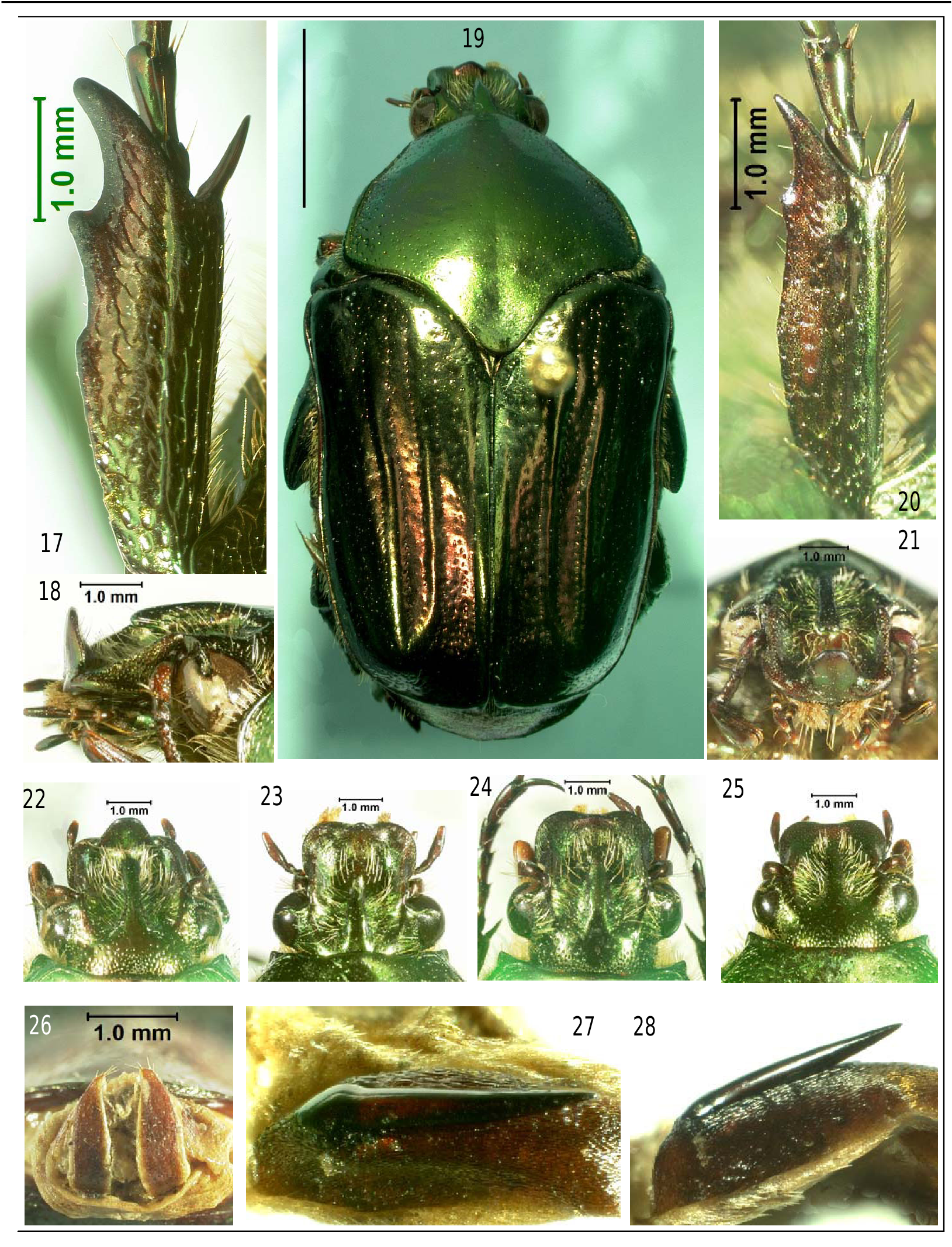
Head (Fig. 9-10, 18, 21-25). Clypeus quadrate, corners rounded, slightly indented below clypeal process, margin not raised. Process or tooth median, triangular in shape, tip rounded, width about one-third of clypeal width, produced upward nearly vertical ( Fig. 18 View Figure 17-28 ), not expanded apically. Head surface finely, shallowly punctate, with central depression margined by raised carinae above eye until clypeus. Central depression containing a narrow frontal process, projecting slightly more than half head length, free beneath for one-fourth its length, narrowing near rounded tip, surface impunctate basally, with few scattered, shallow punctures medially. Process margined by long, fine, anteriorly curved, golden setae in depression. Antennae 10-segmented, club oval, 3-segmented, slightly longer than eye width, lamellae subequal to previous 6 segments, scape with 7 long, fine setae apically. Eye emarginate dorsally for onethird its width, with a fringe of fine, long setae posteriorly.
Pronotum (Fig. 1). With few light punctures centrally, punctures coalescing at sides. Marginal line (or bead) well defined laterally, but absent posteriorly and anteriorly. Anteromedial area slightly prolonged and raised into low conical projection. Medial line (or bead) shallowly depressed in posterior half, cover for scutellum depressed slightly at apex. Metepimeron large, convex, dorsally visible, filling space between posterior pronotal angles and elytra. Pronotum projecting anteriorly into a triangular area (behind eye), visible from above, margined with carinae, creating appearance that pronotum has sharp anterolateral angles Fig. 22-25 View Figure 17-28 ).
Elytra (Fig. 1). Glabrous, flattened, with alternate intervals carinate, surface noticeably punctate. Sutural carina less prominent than 2 central ones, terminating in slight projection (not tooth-like as in C. nitida ), central 2 carinae obvious, terminating posteriorly by fusing at the apical declivity and tumosity, middle carina curved to join lateral one. Elytral termination nearly quadrate.
Pygidium (Fig. 14). Triangular, apex rounded, surface noticeably strigose horizontally throughout, heaviest at base, margined completely. Color basically green, blending to irregular triangular orange area near apex, surface glabrous, without setae on margin.
Legs (Fig. 13). All legs, tarsal segments, and tarsal claws similar in color (as dorsum). Anterior tibia bidentate, with rounded area where 3rd tooth would be (as on female), dorsally with 2 parallel carinae for entire length, anterior tooth sharp, projecting forward. Single terminal spine sharp (as in other tibiae), projecting forward, length subequal to anterior tooth.
Meso- and metatibiae similar, with notch (transverse carina) at posterior third, sharper and slightly better developed in metatibiae. Posterior marginal fringe of long, contiguous, yellow-red setae for entire tibial length. Both apical spurs long, inner about length of first 2 tarsal segments, outer slightly shorter.
Basal tarsomere short, barely half length of second, 2-4 subequal in form and length, 5th longer, slender, terminating in 2 large, fine, sharp, curved claws without teeth or carinae, about length of tarsomeres 2 and 3.
Figure 1-16. Cotinis spp. 1 , 2, 5, 6, 9, 10, 13, 14) Cotinis aliena Woodruff. 3, 4, 7, 8, 11, 12, 15, 16) Cotinis nitida L. 1, 4) Habitus, dorsal. 2, 3) Male genitalia, dorsal. 5, 8) Mesosternal projection. 6, 7) Male genitalia, caudal. 9, 11) Head, dorsal. 10, 12) Head, ventral. 13, 16) Anterior tibia, left, dorsal. 14, 15) Elytral tip and pygidium.
Venter. Medially convex, surface shiny, green (as dorsum), with shallow punctures and few setae on sides of sternites. Terminal sternite lightly punctate on sides, without terminal setae. Mesosternal process rounded at apex (Fig. 5), projecting anteriorly beyond mesocoxae.
Genitalia. Male (Fig. 2, 6, 27, 28). Parameres (in dorsal view, Fig. 2) separated narrowly in apical third, nearly touching terminally. Lateral teeth acute, posteriorly originating from low carinate margin for about half paramere width. Parameres (in caudal view, Fig. 6) terminating nearly horizontally; not abruptly upturned as in C. nitida (Fig. 7). Internal sac ( Fig. 27-28 View Figure 17-28 ) containing a field of fine teeth or spines, into which is imbedded a sharp pointed, heavily sclerotized spine (2 spines present in C. nitida ). A single spine is present in C. viridicyanea also.
Female Allotype. Same data as holotype. Length 22 mm, width 11 mm. Little dimorphism, except anterior tibiae distinctly tridentate ( Fig. 17 View Figure 17-28 ). Genitalia ( Fig. 26 View Figure 17-28 ) consisting of 2 sclerotized plates, orange with yellow borders, separated their entire length and apex with 4 fine setae. Deposited in Florida State Collection of Arthropods.
Paratypes (132). All Florida (numbers of specimens in parentheses): Monroe Co., same data as holotype (84). Additional Islamorada specimens listed chronologically as follows: 23-VI-77, no collr. (1); 27-X-77, W. E. Wyles (1); 9-XII-77, W. E. Wyles (2); 11-XII-77, R. E. Woodruff, W. E. Wyles, bait trap with caproic acid in mineral oil, behind Manny & Isas Restaurant, under Bauhinia tree (1); 9-VI-78, G. Pratt (1); 23-VI-78, W. E. Wyles (6); 12-VII-78, J. Ekblom (2); 18-VII-78, R. Clark (1); 18-VII-78, W. E. Wyles (1); 9-VIII-78, C. F. Dowling (1); 10-VIII-78, E. Tetro (2); 17-VIII-78, P. Chobrda (2); 12-VII-79, L. A. Stange, on Bidens pilosa (1); 19-VIII-80, R. E. Woodruff (1); 3-VIII-83, Mile 88, H. Glenn, on beach (1); 28-VII-94, L. D. Howerton (1); Monroe Co. , Plantation Key, Herbert Zim residence, 1-6-VIII-1979, Paul Tuskes (10); same 30-VII-79 (4), 1-VIII-79 (2), 2-VIII-79(1) .
The most recently collected paratype specimens are from Monroe Co., Big Pine Key, Cactus Hammock , 22-VIII-98, R. Beiriger (5) [“around wild coffee”, pers. comm. of collector]; and Dade Co. , Navy Wells , 22-VII-98, R. Beiriger , in flight (1). The latter represents the first collection on the Florida mainland .
In addition to most of the paratypes deposited in the Florida State Collection of Arthropods , others are deposited in the following collections: Canadian Museum of Nature , National Museum of Natural History , University of Nebraska State Museum , University of Missouri , Illinois Natural History Survey , Ohio State University , California Academy of Sciences , Instituto de Ecología (Xalapa, Mexico), M. A. Goodrich, W. B. Warner, M. A. Morón, C. Deloya, P. E. Skelley, R. Beiriger, and M. L. Jameson .
Variation. In general the type series is uniform in appearance. Length of males varies from 16 to 22 mm; females from 16 to 23 mm. Color varies from green (Fig. 1) to coppery with red reflections ( Fig. 19 View Figure 17-28 ). The greatest variation occurs in the shape of the clypeal process ( Fig. 22-25 View Figure 17-28 ), ranging from prominent to nonexistent. This variation occurs in both sexes. A single specimen from Big Pine Key has the frontal process obsolete, the only vestige being a low, central, vertical tubercle. The pronotal “hump” varies in extent, occasionally with a slight depression posteriorly, producing a low, nipple-like projection.
Comparison. Although not matching, C. aliena specimens will run to couplet 18 in Goodrich’s key (1966): “Brilliant blue-green; elytra weakly bicostate, with rows of usually distinct punctures between costae; posterior sutural angles thickly punctate”... C. viridicyanea Perbosc. A single character will serve to distinguish them: the obvious extensive setal patches surrounding the clypeal process in C. aliena are absent in my only specimen seen of C. viridicyanea . I have included a photo of this rare species (Fig. 32) and commented on it below. They appear similar because of the elytral carinae, and share the single spine in the aedeagus, but they are easily distinguished by specific differences listed in the following paragraphs:
Cotinis aliena: Color bright grassy green, often with red reflections. Smaller: length 16-23 mm. Clypeal beard prominent. Clypeal process pointed, more cylindrical. Overall punctation visible with naked eye. Punctures more numerous, coarser, some coalescing near lateral pronotal and humeral angles. Elytral carinae prominent, more elevated. Pygidium bicolored, basally green, with orange apical third. Scutellum barely exposed. Mesofemora ventrally punctate, covered with setae.
Cotinis viridicyanea (based on the single specimen examined): Color blue-green, with violet reflections (Fig. 32). Larger: length 20.8-28.8 mm (from Goodrich 1966). Clypeal beard absent. Clypeal process flatter, broader, more truncate. Overall punctation noticeable only under magnification, except a few fine punctures barely visible at apex of elytral carinae, appearing shinier. Punctures finer, shallower, rarely coalescing. Elytral carinae less elevated. Pygidium unicolorous as body. Scutellum more exposed. Mesofemora ventrally punctate with few setae.
Immature stages. Pupal cases ( Fig. 29-31 View Figure 29-31 ) consist of rigid cylindrical cocoons composed of relatively coarse sand and calcareous bits of coral and shell. These cases were extremely numerous during a visit in 1988, but none were found to contain pupae. Instead, several mummified adults, presumably killed by a fungus, were found dead inside the cocoon. Within the non-eclosed cocoons the remaining pupal skins were inadequate for description. Dr. Peter Landolt subsequently collected and preserved pupae for study. These and earlier-collected larvae will be formally described and compared with those of C. nitida in a future paper.
Larvae of other Cotinis species have the unusual behavior of surfacing from the soil at night and foraging by crawling on their backs. The speed is remarkable in C. nitida , averaging nearly 50 centimeters per minute ( Hintze 1925). That species is well known for exhibiting thanatosis when touched or disturbed and can survive complete submergence in water for over 48 hours. Because of extensive damage to the lawn at the Ekblom residence, a commercial exterminator chemically treated the lawn and picked up hundreds (“buckets full”) of larvae the following day.
Natural enemies. It appears that the initial populations, reported by nursery personnel and homeowners, subsequently have been greatly reduced. During my last collecting trip to the nursery (Oct., 1979) I encountered thousands of parasitic wasps ( Scolia dubia Say ; det. L.A. Stange) flying about 6 inches above the ground. This wasp has previously been reported as a parasite of C. nitida ( Felt 1933) and other scarabaeid larvae. Such a large wasp population could have diminished the C. aliena population to a low level. The related Euphoria sepulcralis (Fab.) is also common in the area, providing an additional host.
Florida Department of Agriculture nursery inspector W. Eugene Wiles, who collected the first specimen, provided the following observations on blackbird predation. On June 23, 1978, he “saw a flock of blackbirds (probably grackles) fly noisily into a Gumbo Limbo tree, which startled several Cotinis there. The beetles flew immediately to a Sapodilla tree across the street and to other scattered locations. The birds caught several and beat them on the street”. He caught 2 and also observed them copulating in a tree.
Possible origin. Although the first specimens of C. aliena were collected over 30 years ago, this description was delayed initially because it was believed to be an immigrant or recent introduction. After thor- ough checking, comparison with most of the known species, as well as opinions from the major scarab workers, I believe it is undescribed.
There is a narrow possibility that the new species is not native to Florida, even though there is no direct evidence that it is known from elsewhere, or that it has been intercepted in commerce. Many early collectors have worked in the Keys and never obtained specimens. It is such a large, showy beetle it is unlikely that even casual collectors would overlook it. The small islands making up the Florida Keys have been completely under water in recent geological periods, and few true endemic (precinctive) species are known from there. I suspect that the new species has been introduced, and therefore I have given it the name aliena .
Several ideas have been postulated as methods of introduction. The South Florida climate is subtropical, and thousands of landscape plants have been introduced over the past century. Many of these plants originated in Central and South America. The original find, and the most specimens, were collected in a plant nursery. During early surveys, U.S. Customs officials discovered several bales of marijuana that washed up on Islamorada beaches. Such contraband could have provided a suitable substrate for larval food and pupation. The extremely hard and well-constructed pupal cases ( Fig. 29-31 View Figure 29-31 ) could survive such transport. However, we will probably never know how or from where the original specimens arrived.
A similar scenario occurred when another introduced scarab, Plectris aliena Chapin , was discovered in South Carolina ( Chapin 1934). It is now present in Florida and Australia ( Woodruff 1968). All its relatives are South American, but this species has not been discovered there. Chapin chose the name aliena because he believed it was introduced; its origin has yet to be determined after 74 years! Both it and C. aliena inhabit extremely sandy soils, and they could have been transported easily in any of their life stages by commerce or in ballast, soil, or plant containers.
Cotinis aliena was listed as “threatened” in the Florida endangered species list ( Woodruff 1982) and as “rare” in a later classification ( Woodruff and Deyrup 1994). The species was previously listed by Peck and Thomas (1998: 63) as Cotinis n.sp. from “Monroe Co., Islamorada: rare; this species has been collected on only one key; it may be a very localized endemic or it may be an immigrant.” The records above provide little recent information, except that a single specimen has been taken on the Florida mainland (Dade Co.).
| US |
University of Stellenbosch |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |