Marmosa constantiae O. Thomas, 1904
|
publication ID |
https://doi.org/10.1093/mspecies/sew012 |
|
publication LSID |
lsid:zoobank.org:pub:D8F93154-94BE-47A7-A651-A7B46B781F1F |
|
persistent identifier |
https://treatment.plazi.org/id/03E2D47F-FFAE-7064-A3C3-CD4296AC10B1 |
|
treatment provided by |
Felipe |
|
scientific name |
Marmosa constantiae O. Thomas, 1904 |
| status |
|
Marmosa constantiae O. Thomas, 1904
White-bellied Woolly Mouse Opossum
Marmosa constantiae O. Thomas, 1904:243 . Type locality “Chapada, Matto Grosso,” Brazil.
[ Didelphys ( Marmosa) ] constantiae: Trouessart, 1905:856 . Name combination.
[ Didelphis ( Caluromys) ] constantiae: Matschie, 1916:270 . Name combination.
[ Marmosa ( Marmosa) ] constantiae: Cabrera, 1919:36 . Name combination.
Marmosa budini O. Thomas, 1920:195 . Type locality “Altura de Yuto, Rio San Francisco,” Jujuy, Argentina.
Marmosa constantiae budini: Tate, 1933:76 . Name combination.
Marmosa constanciae Mares et al., 1981:161 . Incorrect subsequent spelling of Marmosa constantiae Thomas, 1904 .
M [ icoureus]. constantiae: Gardner and Creighton, 1989:4 . Name combination.
Marmosa (Micoureus) cinerea budini: Anderson, Riddle, Yates and Cook, 1993:14 . Name combination.
Micoureus constantiae budini: Anderson, 1997:9 . Name combination.
Micoureus constantiae constantiae: Anderson, 1997:9 View in CoL . Name combination.
M [ armosa]. constantatiae constantiae de la Sancha et al., 2012:232 View in CoL . Incorrect subsequent spelling of Marmosa constantiae Thomas, 1904 .
CONTEXT AND CONTENT. Order Didelphimorphia , family Didelphidae , subfamily Didelphinae , tribe Marmosini . The subspecies Marmosa c. budini O. Thomas, 1920 was differentiated by size and form. M. c. budini is said to be smaller than the nominate, with smaller teeth, shorter molar toothrows, less pronounced supraorbital processes, and a proportionately longer tail ( Tate 1933), but the description was based on an individual that was “adult, but not old” (O. Thomas 1920:196). M. c. budini was tentatively recognized by Anderson (1997), but the species is now usually treated as monotypic (Gardner and Creighton 2008) pending a revision of the group. M. constantiae is sister to M. regina ( Gutiérrez et al. 2010; de la Sancha et al. 2012). Synonymy is modified from Gardner and Creighton (2008).
NOMENCLATURAL NOTES. Until recently Marmosa constantiae was placed in the genus Micoureus Lesson, 1842 , but multiple phylogenetic studies have found Micoureus to be embedded within Marmosa ( Gruber et al. 2007) . Gutiérrez et al. (2010) and Faria et al. (2013) found the subgenus Micoureus to be monophyletic, but cautioned that the subgenus likely contains unrecognized species, and is in need of revision. Voss and Jansa (2009) returned Micoureus to Marmosa and treated it as a subgenus, emphasizing that this is an interim solution, proposed to preserve the utility of the name Micoureus while conforming to the requirement that genera be monophyletic. The robustness of the Micoureus clade was confirmed by Voss et al. (2014) in a new arrangement for Marmosa that split the genus into 5 subgenera, and retained Micoureus as a subgenus with all its contents.
The generic name Marmosa is derived from the name given to the “murine opossums” of Brazil according to Seba and later adapted to the French as Marmose by Buffon ( Palmer 1904). The name Micoureus probably originated with the Guaraní–Tupi indigenous name for an opossum, Mykuré. The species is named after Mrs. Percy Sladen (possibly Constance) who funded the collecting expedition named in her husband’s honor and during which the type was collected (Braun and Mares 1995).
Didelphys cinerea is listed as a synonym of Micoureus paraguayanus View in CoL (currently Marmosa paraguayana ) by Gardner and Creighton (2008) but the distribution given suggests a composite of species and all localities listed lie outside of the range of M. paraguayana . Thomas (1888) lists various specimens from Bolivia, but the only specific locality mentioned is Santa Cruz de la Sierra which lies only within the range of M. constantiae . D. cinerea was listed as a synonym of M. constantiae by Anderson et al. (1993), but reference to gray-based ventral pelage in the description by Thomas (1888) is not consistent with that species. We omit D. cinerea from the synonymy here on the basis that it cannot be confidently assigned to this species.
Published English common names include pale-bellied woolly mouse opossum (Wilson and Cole 2000; Smith 2011), white-bellied woolly mouse opossum ( Gardner 2005; Gardner and Creighton 2008), and bay-colored mouse opossum ( Mares et al. 1989; Canevari and Vaccaro 2007). The following Spanish language names have appeared in the literature: Marmosa View in CoL grande bayo ( Mares et al. 1989), Comadrejita grande, Comadrejita baya, Comadrejita pálida ( Massoia et al. 2000; Canevari and Vaccaro 2007), Marmosa View in CoL grande baya (Canevari and Vaccaro 2007), Marmosa View in CoL pálida ( Anderson et al. 1993), and Marmosa View in CoL lanuda de vientre claro ( Emmons 1999).
DIAGNOSIS
Marmosa constantiae is a fairly typical member of the Marmosa subgenus Micoureus , with dense, woolly dorsal pelage that is brownish-gray and shorter ventral pelage that is pale buffy-yellow in color. Within subgenus Micoureus , the pale distal one-third of the tail coupled with entirely self-based ventral pelage are diagnostic for M. constantiae . Tate (1933) reported a mammary formula of 7-1-7 which contrasts with 4-1-4 and 5-1-5 reported for other members of the subgenus.
Marmosa paraguayana (Tate’s woolly mouse opossum) is the only other species in the subgenus in which the distal one-third of the tail is consistently and conspicuously paler. M. constantiae can be distinguished from M. paraguayana by a slight reddish-brown tone to the dorsal coloration (most notable laterally) and strongly buffy-yellow ventral coloration. The ventral pelage is basally self-colored in constantiae and graybased in paraguayana as in all other members of the subgenus Micoureus . On direct comparison, M. paraguayana has rather woollier pelage than M. constantiae and the fur does not extend so notably over the base of the tail in the latter ( 2–2.5 cm) as it does in the former ( 3–5 cm). Morphological differences between Paraguayan specimens of M. constantiae and M. paraguayana were elucidated by Smith and Owen (2015). These 2 species are marginally sympatric in northern Paraguay and although paraguayana has an association with Atlantic Forest and M. constantiae with subhumid forest in Chaco and Cerrado areas, the 2 species do occur together in at least 1 locality in the country and may overlap more widely than is currently known (Smith and Owen 2015).
In northern Bolivia, M. constantiae is at least geographically sympatric with Marmosa regina (bare-tailed woolly mouse opossum). It can be reliably distinguished from that species by the extensively pale distal one-third of the tail, distinctly cinnamon dorsal coloration, and self-colored ventral pelage (as opposed to gray-based). Cranially M. regina has a narrower skull and lacks the expanded nasals and palatine fenestrae found in M. constantiae (Gardner and Creighton 2008) .
GENERAL CHARACTERS
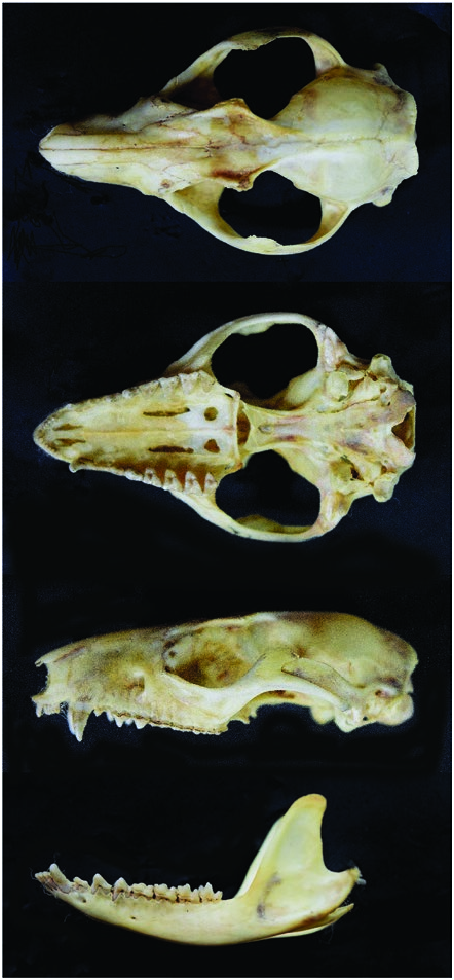
Marmosa constantiae is a large stocky mouse opossum, with a relatively short snout and thick, woolly dorsal pelage ( Fig. 1 View Fig ). Dorsally gray, lightly grizzled due to darker hair bases and paler tips, and with a slight brownish tinge most notable on the flanks. Ventrally buffy-yellow with hairs self-colored to the base, and inconspicuous grayish bases confined to the ventrolateral pelage. In some specimens, the head may be quite strongly buffy-yellow, especially on the cheeks and snout. Black eyerings are prominent and narrow behind and below the eye. The nose is pink, the vibrissae short, and the blackish-purple ears are of moderate size. The tail is long and furred basally for 20–25 mm, being characteristically bicolored with the basal two-thirds a purplish-black and the terminal one-third a pinkish-white (the amount of pale coloration being subject to variation). Thenar and 1st interdigital pads are fused on the hindfoot but lie together on the forefoot. The 4th interdigital pad lies against the hypothenar pad of the forefoot but the 2 are either fused or in direct contact on the hindfoot. Digit IV on the hindfoot is longest with a length ratio of 0.45 when compared to the hindfoot length. Second and 3rd interdigital pads on all feet are triangular and approximately as wide as they are long. No sexual size dimorphism was detected among Paraguayan specimens (Smith and Owen 2015). Description adapted from Tate (1933), Emmons (1999), and Smith (2011). Ranges of external measurements were: length of head and body, 113–180 mm; length of tail, 145–233 mm; length of ear, 20.3–32 mm; length of hind foot, 21–30 mm; mass 35–144 g ( Anderson 1997; Eisenberg and Redford 1999; Flores and Díaz 2002; Cáceres et al. 2007; de la Sancha et al. 2012; Smith et al. 2012; Smith and Owen 2015).
The skull is robust with wide zygomatic arches ( Fig. 2 View Fig ). Round accessory orifices almost always present behind the large posterior palatal foramina. Nasals are broad at the maxillofrontal suture, and broadly rounded posteriorly. In older specimens, the interorbital region is rather broad, and separated by large and pointed supraorbital processes from a postorbital constriction. Palate short and broad, usually with rounded fenestrae behind the posterior palatal foramina (closed in type). Bullae variable, small and often slightly pointed ( Tate 1933; Díaz and Barquez 2002). Ranges of cranial measurements were: condylobasal length, 34.4–42.7 mm; breadth of nasals, 3.0– 6.2 mm; least interorbital breadth, 5.8– 7.8 mm; breadth of zygomatic arch, 19.7–26.2 mm; length of palate, 19.1–23.9 mm; breadth of palate, 12.1–14.4 mm; length of maxillary tooth row, 14.3–17.1 mm; length of molar row, 7.7–8.9 mm; M1–M3, 6.4–7.7 mm ( de la Sancha et al. 2012; Smith et al. 2012; Smith and Owen 2015). Flores (2003) provides a detailed comparative account of cranial morphology for Argentine specimens.
DISTRIBUTION
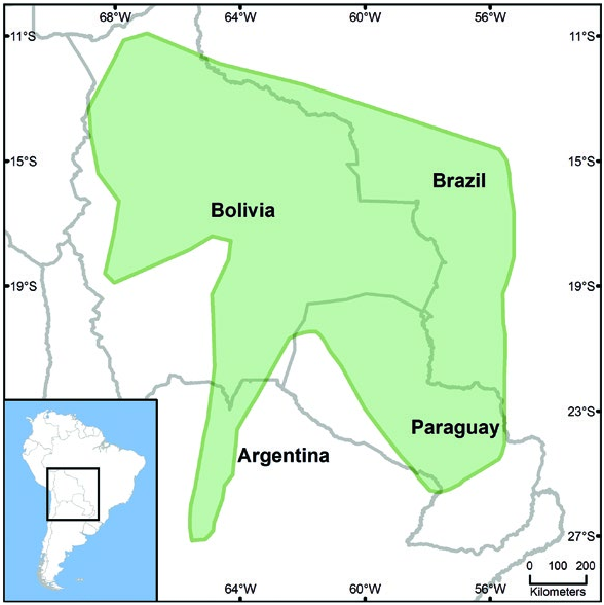
Marmosa constantiae is restricted to central South America ( Brown 2004), where it is relatively widespread from northern Bolivia south to extreme northern Argentina, and east to Paraguay and west-central Brazil ( Fig. 3 View Fig ). In Bolivia, it has been recorded in departamentos Pando, Beni, La Paz, Cochambamba, Santa Cruz, Chuquisaca, and Tarija ( Anderson 1997). M. constantiae is little known in Brazil where it has been reported only in Mato Grosso, Mato Grosso do Sul, and Rondônia states (Melo and Sponchiado 2012). The Argentine distribution includes provincias Jujuy (Díaz and Barquez 2002; Gamboa Alurralde et al. 2015), Tucumán ( Flores and Díaz 2002), and Salta ( Mares et al. 1989; Díaz et al. 2000), with a single record of a specimen from the Chaco region in Provincia Formosa ( de la Sancha et al. 2012). M. constantiae has only recently been reported for Paraguay (Voss et al. 2009) but is apparently fairly widespread in the Cerrado and Chaco regions with specimen records from both sides of the Paraguay River in departamentos Amambay, San Pedro, Presidente Hayes, and Alto Paraguay. It was erroneously reported from Departamento Boquerón ( de la Sancha et al. 2012), although it may occur there ( Smith et al. 2012; Smith and Owen 2015) and also potentially occurs in Departamento Concepción. Specimens of M. constantiae from Parque Nacional Defensores del Chaco in Departamento Alto and all with slightly spatulate crowns. Last upper molar compressed. P2 and P3 of similar size and larger than P1. Canines are well-developed ( Tate 1933; Díaz and Barquez 2002).
Paraguay are morphometrically distinct from other Paraguayan M. constantiae specimens and may represent an unrecognized taxon (Smith and Owen 2015). No fossils are known.
FORM AND FUNCTION
Marmosa constantiae shows numerous adaptations for an arboreal lifestyle. Tail scales are rhomboid and arranged in a spiral, and the tail is sparsely haired and prehensile. Feet are broad, the stout claws of the forefeet extending slightly beyond the digital pads as an adaptation for climbing. The median part of the soles of the feet is smooth, but the ventral surfaces of the digits have transverse bars as an aid for gripping. The flexor tendons of the manus of this species were described in a comparative study by Abdala et al. (2006). Males possess bony radial tubercles that are absent in females, and it is assumed that these perform a copulatory function (Lunde and Schutt 1999).
Females lack a marsupium but have 15 inguinal mammae arranged in a circular pattern (7-1-7—Gardner and Creighton 2008). The abdominal-inguinal mammary field is pigmented ochraceous in lactating females ( Flores et al. 2000). The male has been stated to have a pink scrotum ( Emmons 1999), but 1 male from Departamento San Pedro, Paraguay, had a bluish scrotum ( Smith et al. 2012). Males lack a gular gland ( Hershkovitz 1992).
Dental formula of adults is i 5/4, c 1/1, p 3/3, m 4/4, total 50. A slight diastema is present between I1 and I2 and I5 is always slightly larger, sometimes separated from I4 by a very slight space. Mandibular incisors are semirecumbent, closely appressed
ONTOGENY AND REPRODUCTION
Of 6 female Marmosa constantiae taken in Bolivia, 4 had no embryos in July, August, and September, 1 was lactating in May, and 1 had 5 young in August ( Anderson 1997). Tate (1933) notes breeding or nursing females in January (presumably in Bolivia) and juveniles in April. In Provincia Jujuy, Argentina, a lactating female was taken in June ( Flores et al. 2000) and a juvenile was taken in August ( Flores 2006).
None of the specimens captured during July, August, and February in Departamento San Pedro, Paraguay, showed signs of reproductive activity ( Smith et al. 2012), but a female was captured with 7 hairless young in September 2012 at the same locality. Young were anchored to the teat with their mouth and though they were able to use their legs to move, they lacked sufficient strength to hold onto the pelage of the mother. On the day of capture, the young had the following mean measurements ( n = 6): length of head and body, 30.3 mm; length of tail, 6.3 mm; mass; 1.8 g. Three days later these had increased to ( n = 7): length of head and body, 31.3 mm; length of tail, 12.9 mm; mass, 2.1 g. Young fed from a specific teat, ignoring other available teats and were not aided by the mother when reattaching. After 6 days in captivity the female consumed all the offspring. The same female had been captured during May of the same year when it had a mass of 62 g, increasing to 70 g upon recapture a month later and 85 g after consuming its young (September).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Marmosa constantiae O. Thomas, 1904
| Smith, Paul & Owen, Robert D. 2016 |
Micoureus constantiae budini :
| ANDERSON, S. 1997: 9 |
Micoureus constantiae constantiae :
| ANDERSON, S. 1997: 9 |
Marmosa (Micoureus) cinerea budini :
| ANDERSON, S. & B. R. RIDDLE & T. L. YATES & J. A. COOK 1993: 14 |
Marmosa constanciae
| MARES, M. A. & R. A. OJEDA & M. P. KOSCO 1981: 161 |
Marmosa constantiae budini :
| TATE, G. H. H. 1933: 76 |
Marmosa budini O. Thomas, 1920:195
| THOMAS, O. 1920: 195 |
Marmosa ( Marmosa )
| CABRERA, A. 1919: 36 |
Didelphis ( Caluromys )
| MATSCHIE, P. 1916: 270 |
Didelphys ( Marmosa )
| TROUESSART, E. L. 1905: 856 |
Marmosa constantiae O. Thomas, 1904:243
| THOMAS, O. 1904: 243 |