Desmodema Walters & Fitch 1960
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5039.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:234D03A3-1AC7-442E-A8A5-784EB3EE4394 |
|
persistent identifier |
https://treatment.plazi.org/id/03E29102-FFB6-FFA6-C78F-37AB4923ACB1 |
|
treatment provided by |
Plazi |
|
scientific name |
Desmodema Walters & Fitch 1960 |
| status |
|
Desmodema Walters & Fitch 1960 View in CoL View at ENA
Figures 23 View FIGURE 23 , 24 View FIGURE 24 , 26–29 View FIGURE 26 View FIGURE 27 View FIGURE 28 View FIGURE 29
Type species. Trachipterus jacksoniensis polystictus Ogilby 1898 , Newcastle , New South Wales, Australia .
Material Examined. Species-specific material listed below. Desmodema spp. : HUMZ 113370 View Materials (273 mm SL, Pacific , Japan, Hokkaido) ; HUMZ 186227 View Materials (942 mm TL, Pacific , Japan, off Miyagi) ; NSMT-P91125 (63.9 mm SVL, Pacific, Japan, Kyushu , Kagoshima Prefecture)
Diagnosis (Adults). Body long (to 1100 mm SL), laterally compressed, tapering to a thin, exceedingly elongate caudal peduncle posterior to the anus; whiplike caudal region (narrower than in Trachipterus ; exceedingly more elongate than Zu) curved dorsally. Snout-vent length about 1/3 to 1/4 SL, relative SVL decreasing with increasing SL. Dermal tubercles and pore system present throughout trunk, tubercles on ventral edge of body do not project beyond the general body surface as in Trachipterus . Slight to strong dorsal curve of the body margin in the trunk/ caudal region posterior to anus. Scales cycloid, with ridges or with 1 to 4 spinous ridges ( D. lorum ). Lateral line runs the length of the elongate caudal region and ends at the caudal base. Lateral-line plates with 1 or 2 spines that are much smaller than in Trachipterus or Zu. Relative to the lateral-line scale, the spines point laterally (not angled anteriorly as in Trachipterus ). Body depth at pectoral fin 7.2 to 17.9 % SL, decreasing with increasing length; body depth at anus 5.4–13.3 % SL, decreasing with increasing length. Seven pterygiophores anterior to first neural spine, 1 or 2 between first and second neural spine. Teeth on premaxilla 1–4, with one or two in the form of large, recurved fangs; 2 (4 in NSMT 57647) large recurved fangs on the dentary; vomerine teeth variably present. Gill rakers on the first arch 2–4 +7–10, most commonly 3+9, spinules variably present on upper arch rakers. Pseudobranch well developed, inner operculum strongly pigmented at the gill margin. Dorsal-fin rays 116–215, anteriormost 5–6 rays reduced to a dorsal ridge (i.e., a bony process representing the pterygiophores of the anteriormost elongate dorsal-fin rays of juveniles) that originates over the preopercle; first few fin rays relatively short, the subsequent rays increasing in length, reaching a maximum length over and slightly posterior to the anus; fin rays on the whiplike caudal region gradually decreasing in length. Pectoral-fin rays 1+ 10–14 (one exceptionally short fin ray followed by 10–14 rays). Pelvic fins absent, bony bases not present as in adult Trachipterus and Zu; skin growing over slit-like opening where elongate pelvic-fin rays of juveniles existed (more obscured than in Zu and Trachipterus ). Caudal-fin rays 6–8, typically 7; no ventral caudal lobe is present and all rays are supported by the terminal centrum; caudal fin parallel to the caudal peduncle (not dorsally perpendicular as in Trachipterus and Zu). Minute lateral spinules on the dorsal-, pectoral-, and caudal-fin rays greatly reduced or absent. Anal fin absent. Anus most commonly situated on the left side, occasionally on the midventral line. Total vertebrae 71–77 ( D. polystictum ) or 106–111 ( D. lorum ).
Color. Body primarily silver, dark brown or black dorsally. Dorsal-fin rays bright red or crimson except those in the whiplike caudal region, as well as the caudal-fin rays, which are black. Pectoral fin clear or black. Anterior profile, front of snout, tip of mandible to the anteriormost dorsal-fin ray black. Based on D. polystictum (see Remarks).
Remarks. Specimens of Desmodema are reported as chocolate brown by Harrisson & Palmer (1968). However, Rosenblatt & Butler (1977) attributed this to preservation, with D. lorum much darker than D. polystictum when preserved. Fresh specimens or images of fresh specimens of D. lorum are not available and the color of adults in life is unknown. It is hypothesized that D. lorum is overall darker in color because of a deeper, darker habitat than D. polystictum .
The presence of a short first pectoral-fin ray has not been previously reported. Haemal spines of the posterior most vertebrae (last 10 or so) may pierce through the body wall, most likely as a result of the dorsoventral constriction associated with the caudal region or due to the fragility of this region and subsequent damage during collection. These protruding haemal spines are frequently mistaken for anal-fin rays. Unlike other trachipterids, there is no ventral caudal lobe in Desmodema . All caudal-fin rays originate from the terminal centrum and its fused hypural; the ventral hypural is rayless (see Rosenblatt & Butler 1977: fig. 1).
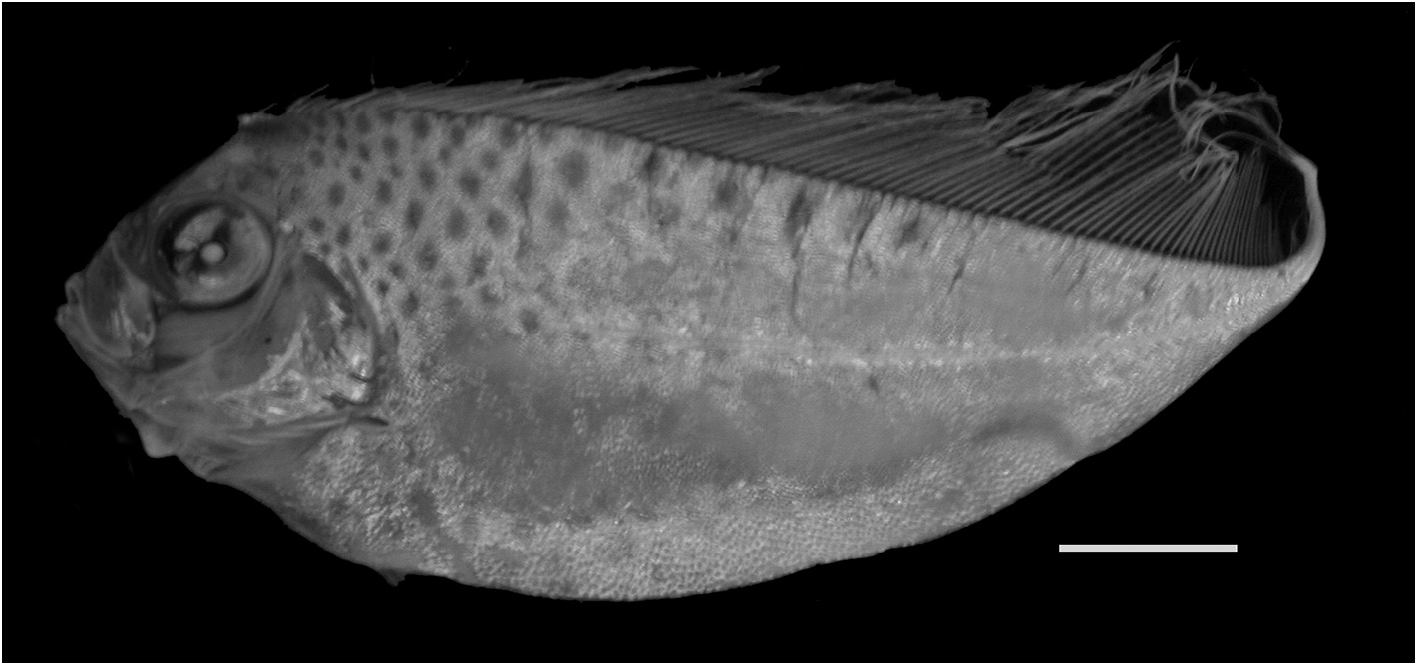
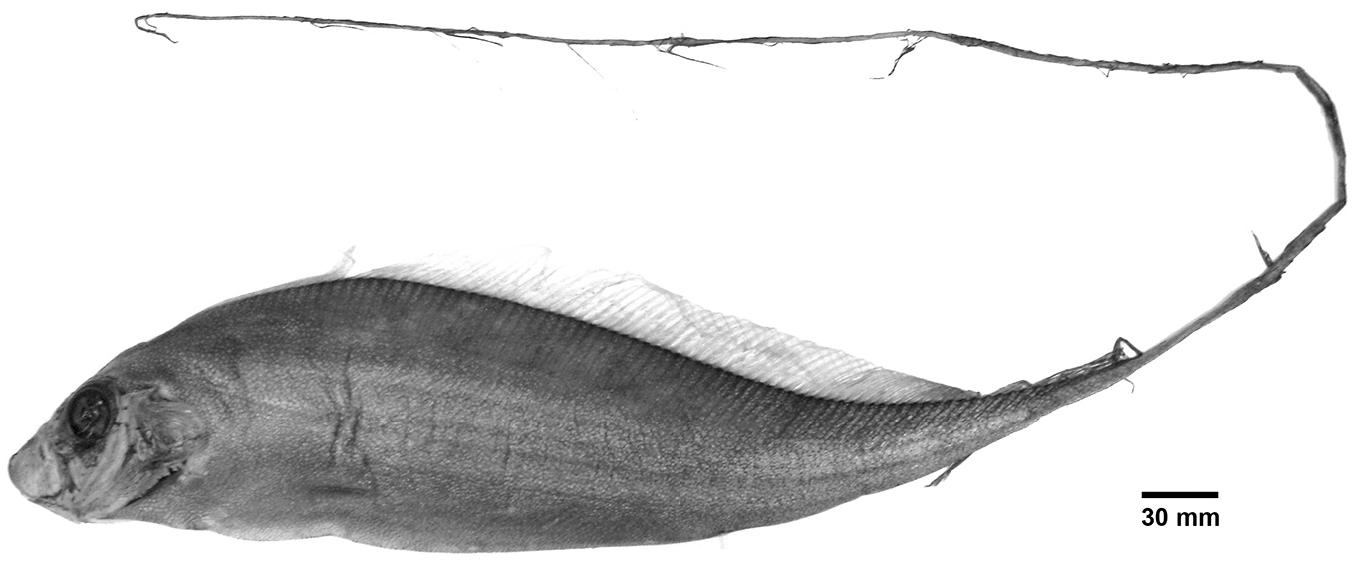
Notes on juveniles. Compared to adults, juvenile Desmodema are profusely spotted, deeper bodied, have a relatively shorter postanal length, and, depending on the stage (early vs late), elongate anteriormost 5–6 dorsal-fin rays and pelvic-fin rays may or may not be present. In young juveniles ( Fig. 23 View FIGURE 23 ) the body outline is almost triangular, with the maximum body depth at the pelvic fin. As body depth decreases, the dorsal-fin ray length increases until just anterior of the caudal peduncle. The 5–6 anteriormost elongate dorsal-fin rays, referred to as the dorsal pennant by Rosenblatt & Butler (1977), are present (see Amaoka et al. 1992: fig. 23). Pelvic fins are also present and the fin rays are extremely elongate, exceeding the body length. These elongate dorsal- and pelvic-fin rays are flat and fan-like in appearance and lack any ornamentation (as in Zu).
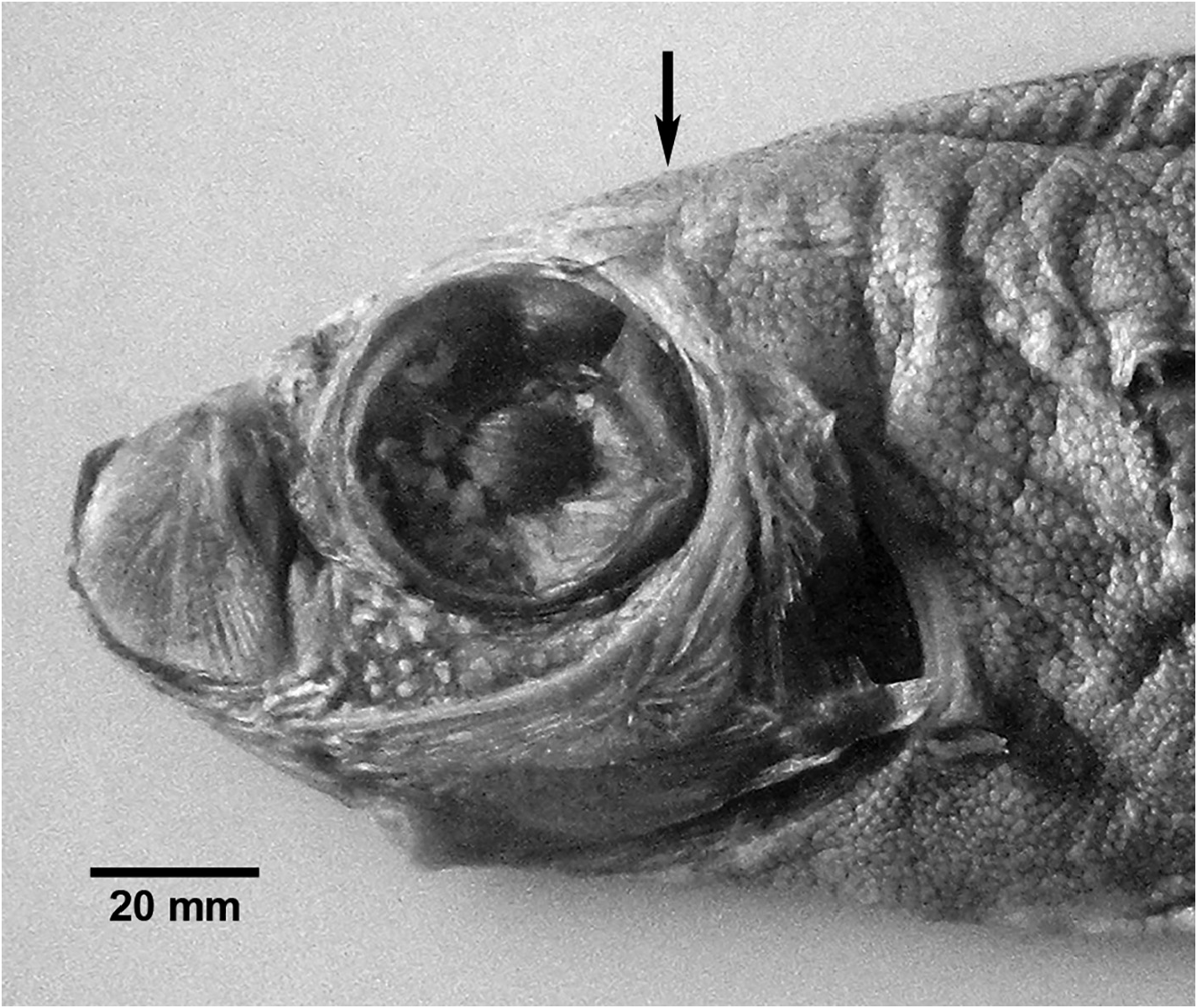
As individuals grow the body outline becomes more rounded. The dorsal pennant begins to reduce in length, ultimately represented by a bony ridge of pterygiophores in adults ( Fig. 24 View FIGURE 24 ). The elongate pelvic fins are absent, but a small slit-like opening at the position of the pelvic fin may be present. This heals over completely in adult specimens. The loss of pelvic-fin rays appears to happen to the bases of the pelvic fins (unlike Trachipterus where reduced pelvic-fin rays protrude from the body wall), and is hypothesized to happen relatively quickly as proposed by Rosenblatt & Butler (1977). Observations of the material examined for this study support this hypothesis as no specimens showed an intermediate stage in which there are reduced pelvic-fin ray lengths (in which fin ray breakage is not obvious). Also, pelvic-fin ray stumps (as found in Trachipterus and Zu) were not identified. The whip-like tail extension is thin and relatively short in small juveniles, greatly increasing in length relative to the SVL. The dorsalfin rays present in the tail region are short in comparison to those present in the trunk region of the dorsal fin. A gas bladder is present in juveniles to about 300 mm ( Rosenblatt & Butler 1977).
Color. Juveniles are characteristically silvery and profusely spotted. The spots are typically larger dorsally, more abundant in the dorsal and anterior regions than ventrally and posteriorly and are reported as bluish to blackish in freshly collected specimens. Spots are black in preserved specimens. Dorsal, pelvic, and caudal-fin rays are red (coloration typically lost during preservation). If elongation of the whip-like caudal region has begun, then the dorsal-fin rays in this region are black and the caudal-fin rays may be either red or black.
Ontogeny (juvenile to adult). There is a protracted period of transition from the juvenile stage to the adult and this transition does not appear to be correlated solely with size. Rosenblatt & Butler (1977) termed one stage of transition in this period, in which rapid morphological changes are hypothesized to occur, as a metamorphosis. The following changes occur throughout development from juvenile to adults, typically in the order listed, although some overlap in relative timing exists: 1) body shape from triangular to teardrop; 2) body depth from deepest at the head, shifting posteriorly to roughly 1/3 SVL; 3) loss of the dorsal pennant; 4) loss of the pelvic fins; 5) elongation of the whip-like caudal region; 6) loss of spotting; and 7) caudal and dorsal-fin rays on the whip-like caudal region change from red to black.
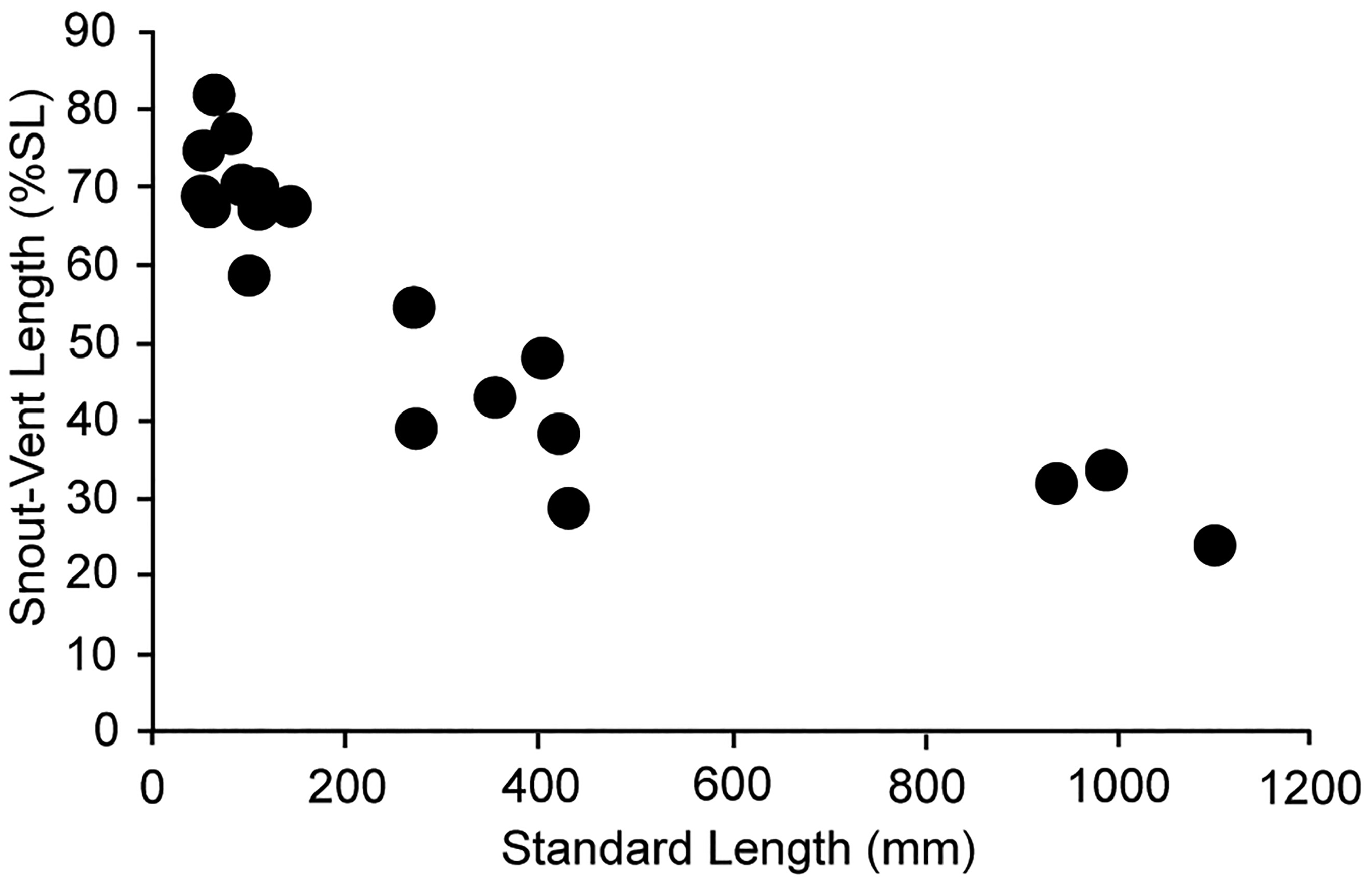
In early juveniles, the anteriormost dorsal-fin rays (typically 5, sometimes 6) are extremely elongate, and the complete reduction of these rays results in the dorsal ridge present in adults. Pelvic-fin rays are also elongate in young juveniles and reduced to their bases in adults. New material and both still photos and video of live juvenile specimens in their natural habitat (which were unavailable to Rosenblatt & Butler 1977) suggest that these reductions may occur more gradually then previously suggested. Juvenile specimens in videos (in which damage due to collection has not occurred) filmed off Japan have functioning dorsal- and pelvic-fin rays, which are intermediate in length. Reduction of these fin rays has begun but elongation of the caudal region has not. Therefore, it appears that the elongation of the caudal region does not begin until the loss of the elongate dorsal and pelvic-fin rays commences and continues after the loss of elongate fin rays is complete. In specimens between 50 to 142 mm SL, SVL ranges from 58–82% SL. This decreases to 29–54% SL in specimens 271–430 mm SL and in specimens ranging from 935 to 1098 mm SL, SV is 24.2–33.6 % SL ( Fig. 25 View FIGURE 25 .). The elongate whip-like caudal region acts to extend the length of lateral line, which reaches its posterior tip.
It is hypothesized that the transition from juvenile to adult is more rapid for Desmodema than in either Trachipterus or Zu, but it is not likely to happen as fast as predicted by Rosenblatt & Butler (1977). A more gradual transition appears to occur with these characters. Abrupt changes that would suggest a complete and immediate loss do not appear to describe ontogeny of Desmodema . However, additional complete specimens in these transitional stages need to be examined.
Significant color changes occur during the juvenile to adult transition. Caudal and dorsal-fin rays along the caudal region are red in young juveniles. With progression into the adult stage these fin rays change to a deep black. This typically occurs after the loss of the elongate dorsal and pelvic-fin rays and elongation of the caudal region, however, the blackening of the caudal-fin rays may occur prior to any reduction in elongated rays or tail extension. The loss of the polka-dotted spotting is the most gradual of the morphological transitions and the last to be complet- ed. Spots, which are not all uniformly sized, are lost in posterior-to-anterior (NSMT 57555), and ventral-to-dorsal (NSMT 91459) directions. Rosenblatt & Butler (1977) reported a 95 mm SV specimen (species unknown) as their largest with polka-dotted spotting pattern. In the current study, a complete (full-body) polka-dotted spotting pattern was observed in a 147.8 mm SVL (271 mm SL) specimen of D. polystictum (NSMT 63975); a 125.4 mm SVL (430 mm SL) specimen of D. lorum (NSMT 57555) retained an abundance of polka-dotted spotting anteriorly.
Notes on eggs and larvae. In their review of lampridiform genera, Walters & Fitch (1960) stated that eggs and early larvae of Desmodema were unknown (see also Olney 1984). Charter & Moser (1996) described eggs and larvae of D. lorum from the northeastern Pacific Ocean. Regarding D. polystictum, Walters & Fitch (1960) , Olney (1984), Olney & Richards (2006), and Fahay (2007) all reiterated that eggs and early larvae were unknown. However, Pertseva-Ostroumova & Rass (1973) described thirteen eggs and two early larvae identified as D. polystictum from the southeastern equatorial Pacific Ocean. These specimens were not available for examination for the current study, but based on the illustrations (see Pertseva-Ostroumova & Rass 1973: fig. 26) and morphological descriptions, this identification is supported. The eggs and larvae of Desmodema differ from Zu and Trachipterus based on the number of melanophores scattered throughout the yolk (much greater in Desmodema ) and by having roughly 70 myomeres. This corresponds with myomere counts reported for D. polystictum ( D. lorum with 102–112; Charter & Moser 1996; of other trachipterids, only Z. cristatus and some species of Trachipterus have approximately 70 myomeres). Additionally, the distribution for D. lorum is restricted to the northern Pacific Ocean. Okiyama (2014) described and illustrated a 27.5 mm SL specimen of D. polystictum . Recently, two eggs that were collected off the northwestern coast of Sri Lanka were identified as D. polystictum morphologically and through DNA barcoding ( Rathnasuriya et al. 2019). These eggs were collected temporally and geographically close to large numbers of adult specimens (99 individuals over two sampling stations) that were taken in nighttime trawls of the upper 30 m of the water column, suggesting that this portion of the Central Indian Ocean is a spawning area for D. polystictum . Regardless, early life history information for any species of Desmodema is very limited.
Planktonic eggs are large and range from 2.3–2.7 mm in diameter ( D. lorum 2.4–2.5 mm; D. polystictum 2.3–2.7 mm). They have a homogenous yolk ranging in diameter from 2.0– 2.4 mm ( D. lorum 2.1–2.3 mm; D. polystictum 2.0– 2.4 mm) and no oil globule ( Charter & Moser 1996: fig. 1; Pertseva-Ostroumova & Rass 1973: fig. 26; Rathnasuriya et al. 2019: fig. 2). Eggs of lampridiform fishes are reported as being brightly colored either pink, red, or amber ( Olney 1984). An amber to pink chorion is reported for D. lorum ( Charter & Moser 1996) , however, the English translation of Pertseva-Ostroumova & Rass (1973) makes no mention of any pink or red coloration to the egg. Similarly, the images published by Rathnasuriya et al. (2019: fig. 2) do not show any reddish coloration, nor does their description make any mention of this coloration. Pigmentation patterns described for the eggs and embryos for both species are similar with melanophores scattered on the yolk and two rows of melanophores dorsally on the epaxial myomeres. Eggs are epi- to upper mesopelagic and have been collected from 0– 100 m.
Hatching length is reported as <6 mm. Pertseva-Ostroumova & Rass (1973) were able to incubate a later stage egg for seven days and at hatching the larva was 5.1 mm (after preservation). The first dorsal fin-ray of this larva was elongate and had two pigmented swellings and three shorter rays posterior to it. The pigment pattern described for a 6.2 mm larva of D. polystictum damaged during collection (the ends of the dorsal and pelvic rays were broken) consisted of accumulations of melanophores on the forebrain, ascending process of the premaxilla, the lower jaw, gut and the base of the pelvic fins. On the caudal fin there were three large concentrated spots along the dorsal edge and two spots on the ventral portion. ( Pertseva-Ostroumova & Rass 1973). Preflexion pigmentation described for D. lorum is similar except for the lack of concentrated spots in the dorsal and ventral regions of the tail ( Charter & Moser 1996).
Flexion for Desmodema is reported to occur at greater than 11.3 mm ( Charter & Moser 1996) followed by development of the caudal and, lastly, the pectoral-fin rays. Postflexion pigmentation patterns for D. lorum show an increase of pigmentation on the head, over the brain and around the eye, laterally on the body and on the dorsal pterygiophores. The juvenile polka-dotted spotting pattern is not apparent in D. lorum of roughly 25 mm ( Amaoka et al. 1992) but a complete pattern is attained by 52.3 mm SL (USNM 164325).
Myomere counts for D. lorum are 106–112. Total myomere counts for the two larvae described as D. polystictum ( Pertseva-Ostroumova & Rass 1973) were 70 and 72 +? (the last few were not counted). Along with egg morphometric data and the pigmentation patterns, these counts suggest that these larvae are representative of D. polystictum .
Ontogeny and habitat. As with other trachipterids, morphological changes throughout development correspond with changes in habitat association as larvae and juveniles transition from a planktonic existence in the euphotic zone into deeper, more offshore waters. Specimens with elongate dorsal- and pelvic-fin rays are observed in the epipelagic zones. Spotted juveniles are taken at or near the surface where their pigmentation pattern may function as “protective coloration in the light-dappled environment” ( Rosenblatt & Butler 1977: 845) and break up the body shape of the fish. Tanaka (1908) reported the presence of spotted juveniles ( Trachypterus misakiensis = D. polystictum ) with elongate pectoral-fin rays near shore after stormy weather, suggesting an epipelagic habitat and somewhat limited swimming ability. Spotted juveniles have also been captured via nightlight in surface waters ( Trachypterus misakiensis = D. polystictum ; Herre & Harold 1950). Specimens figured by Tanaka (1908) and Herre & Harold (1950) depict spotted juveniles with elongate pelvic-fin rays and greatly reduced anteriormost dorsal-fin rays, supporting the notion that elongate fin ray loss is not simultaneous as proposed by Rosenblatt & Butler (1977). However, capture data suggest that spotted juveniles may have a greater depth range. Numerous spotted juveniles have been collected in stomachs of lancetfish ( Alepisaurus spp. ) that were caught on longlines set between 150–305 m ( Fitch 1964; Fourmanoir 1969; Rosenblatt & Butler 1977; however, the lancetfish prey may not have been eaten at the same depth where the lancetfish were caught), 113 mm SL specimen from the vicinity of the Line Islands in the central Pacific was captured in an open-net tow to about 100 m ( King & Ikehara 1956), and the first record of D. polystictum from Oman ( Fig. 26 View FIGURE 26 ), a juvenile in which spots are not visible, was collected at 1000 m by a deep-water trawler (Laith Jawad, pers. comm.).
Rosenblatt & Butler (1977) suggested that a loss of the spotting pattern, elongation of the caudal region and loss or reduction of a gas bladder coincide with a change in habitat as individuals move offshore and adapt to an assumed vertical orientation, as in other members of Trachipteridae . However, vertical orientation is exhibited even at very young ages (larvae prior to the development of spotting patterns with extremely elongate dorsal and pelvic-fin rays, and spotted juveniles) in nearshore surface habitats as evidenced by videos and photographs of live larvae and juveniles filmed from eastern Japan (e.g., http://dailyomi.blog.fc2.com/blog-entry-169.html?sp). Adult D. polystictum (with a dorsal ridge, complete loss of the pelvic-fin rays, elongate caudal region and pigmentation changes consisting of loss of spotting and black vs. red dorsal and caudal rays in the elongate caudal region) are collected in offshore water to roughly 500 m ( Rosenblatt & Butler 1977). At least one instance of an adult D. polystictum swimming vertically in shallow (5–6 m) water has been reported from Guadeloupe, French West Indies ( Fig. 27 View FIGURE 27 , Daniel Rabbe, pers. comm). This individual has all typical adult characters consisting of complete loss of first 5–6 dorsalfin rays, complete loss of pelvic-fin rays, elongation of the caudal region, loss of spotting and blackening of the fin rays on the elongate caudal region. Specimens matching this description are typically found in deeper, offshore water, and therefore this record is a unique occurrence.
Taxonomic history. In 1897, a rare fish washed up on the beach at Newcastle, New South Wales. The specimen was passed along to the Australian Museum’s ichthyology curator, J. Douglas Ogilby for identification. At the time of Ogilby’s description of the individual, only eight related specimens were known from Australasian waters and Ogilby recognized this ninth as a young trachipterid (140 mm SL). Ogilby (1898: 656) noted the differences between this specimen and the other described Australasian specimens, such as fin-ray numbers and morphology, head profile, body contour, and proportions. Regarding its specific identity, he states “…these fishes pass through many and puzzling changes in their passage from youth to maturity, and recognizing, therefore, the necessity for exercising the greatest caution in dealing with specimens of different ages but from neighboring localities, it is equally incumbent on us to guard against falling into the opposite error by carelessly uniting together…what may prove to be very distinct species.” While clearly aware of potential ontogenetic and geographic variations and unable to fully align this individual with other Australasian representatives, Ogilby made the decision to rank this specimen as a subspecies of Trachypterus jacksonensis ( Ramsay 1881) based primarily “on account of the numerous spots which ornament the head and body”. The coloration of this specimen varied significantly from all ontogenetic stages of all other species known at the time.
In revising the suborder Trachipteroidei, Walters & Fitch (1960) erected the genus Desmodema for the placement of Trachypterus jacksoniensis polystictus Ogilby 1898 . Walters & Fitch (1960) differentiated the genus Desmodema from Zu based, in part, on 1) the orientation of the ventral profile (straight vs. scalloped) and 2) the lateral line on the caudal region (straight vs. wavy), and from Trachipterus based on 1) the number of vertebrae (104–109 vs. 69–101), 2) the presence vs. absence of scales and 3) orientation of the dorsal caudal-fin rays relative to the caudal peduncle (parallel vs. angled dorsally). Walters and Fitch (1960) referenced an unknown number of specimens presumably ranging from late larvae to adult; neither the ontogenetic nor the geographical variation represented by their generic diagnosis is known.
In review of the trachipterids from the eastern Pacific, Fitch (1964) examined 26 Pacific specimens of D. polystictum and based his redescription of the species on 12 individuals from the eastern Pacific (11 mm – 1106 mm SL). Although Fitch (1964) acknowledged the morphological changes that occur with ontogeny, his description did not specifically address these changes. However, Fitch recognized both T. misakiensis Tanaka 1908 and T. deltoideus Clark 1938 as conspecifics of D. polystictum and synonymized them accordingly.
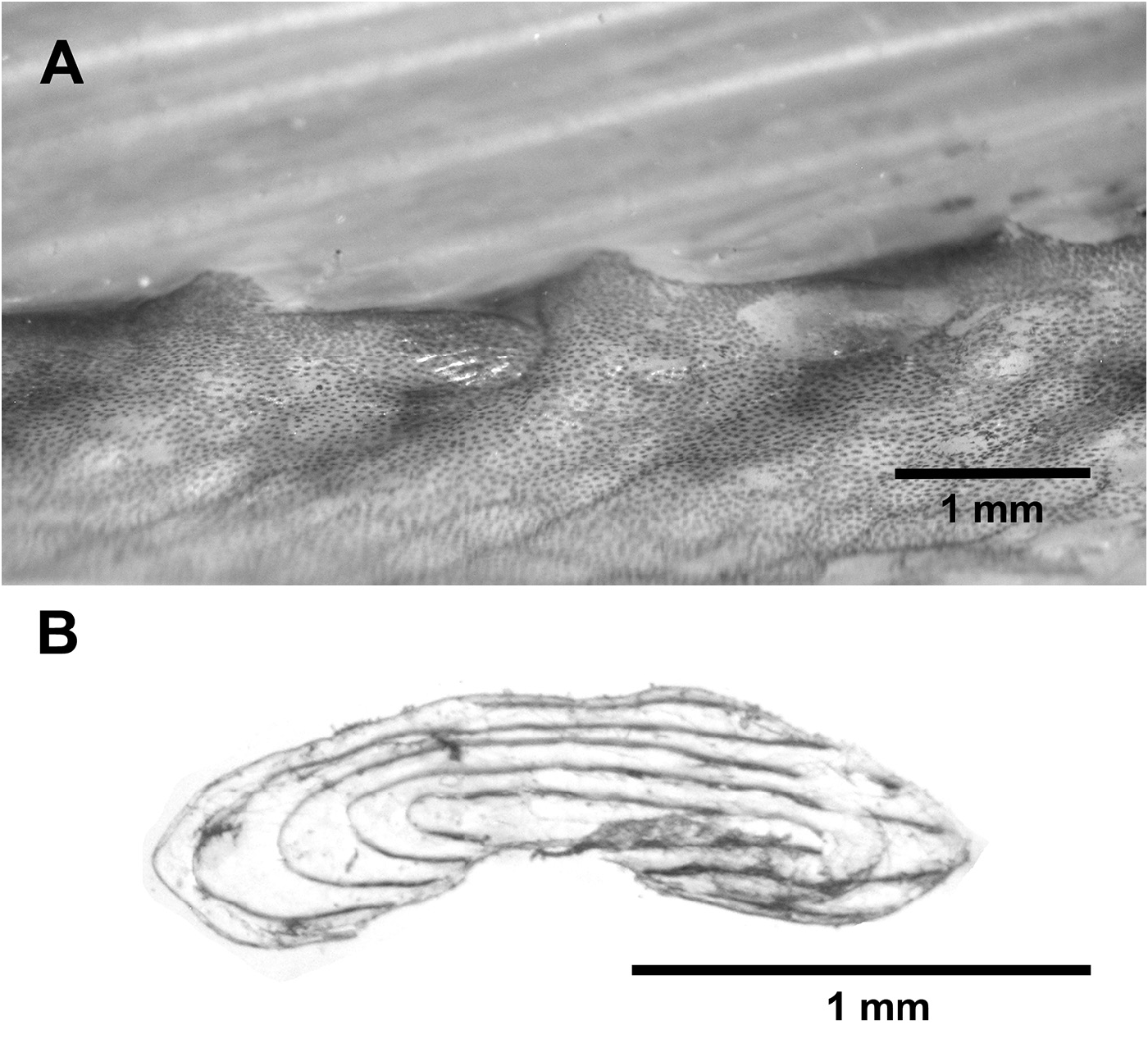
A second species of Desmodema was described by Rosenblatt & Butler (1977) with their description of D. lorum from the northern Pacific. These authors addressed previous confusion in the literature regarding the presence of scales in the genus. For example, there is no mention of scales by Ogilby (1898) in the original description and Tanaka (1908) noted that the body is scaleless and smooth. However, Walters & Fitch (1960: 446) and Fitch (1964) noted that the body of Desmodema is covered with “…non-imbricated elliptical scales each with two slightly divergent spinose ridges.” Rosenblatt & Butler (1977) described D. polystictum as scaleless at all sizes and the young of D. lorum being covered with scales, each with a pair of longitudinal spinous ridges, and adults as scaleless. Confusion regarding the presence of scales continued to persist, as Zacharia & Kannen (2012) described an adult specimen of D. polystictum as scaleless. Examination of new material (this study) reveals the presence of scales in juveniles and adults in both species of Desmodema . Non-overlapping, cycloid scales are located on at the base of the dorsal-fin rays in D. polystictum from the northwestern Atlantic (MCZ 60557 355 mm SL, Fig. 28 View FIGURE 28 ). However, a northwestern Pacific specimen (NSMT 91459, 94.3 mm SVL) was covered with slightly overlapping scales that bear longitudinal ridges and do not alter the pigmentation pattern when removed (note that these ridges are not the same as those found on the lateral surface of some ctenoid scales, i.e., some spinoid scales; Roberts, 1993: fig. 2C). A second northwestern Pacific specimen of D. polystictum (NSMT 68656, 140.5mm SVL) has large patches of simple (i.e., without ridges) cycloid scales covering the body.
Juvenile and adult specimens of D. lorum have cycloid scales; although these are both with and without ridges (NSMT 58740, 404 mm SL) and may be rectangular. Scales may have 1–4 ridges either with or without spines (USNM 164325, 60 mm SL; HUMZ 113370, 273 mm SL). These differences in scale morphology do not appear to correlate with geography, ontogeny, or morphological placement. No scales with spines were observed in D. polystictum in the current study.
Rosenblatt & Butler (1977) provided a detailed redescription of Desmodema , but nearly all specimens they examined were from the northeastern Pacific Ocean, thereby masking potential geographical variation. Additionally, most specimens were damaged and no material smaller than 18.9 mm SV were examined. Although the authors addressed some ontogenetic changes (juvenile to adult), character changes associated with the larva-to-juvenile transition were not discussed.
Species-level diversity. Since the establishment of the genus by Walters & Fitch (1960), Desmodema has been reviewed by Fitch (1964) from the eastern Pacific, globally by Rosenblatt & Butler (1977), Heemstra & Kannemeyer (1984) from South African waters and Ji et al. (2009) from Korean waters. The genus was considered monotypic until the work of Rosenblatt and Butler (1977) in which they described D. lorum ; prior work was unknowingly based on both species.
The following summarizes morphological differences between D. polystictum and D. lorum . Data are synthesized from published work and from new specimens, some of which represent new geographic locations, examined in this study.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.