Pericelis flavomarginata, Tsuyuki & Oya & Jimi & Kajihara, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4894.3.6 |
|
publication LSID |
lsid:zoobank.org:pub:E38ECAF6-C41A-4BF3-8F00-7166E0A61FA1 |
|
DOI |
https://doi.org/10.5281/zenodo.4323927 |
|
persistent identifier |
https://treatment.plazi.org/id/03E287BE-C060-934A-FF27-CAF2FB37D8AB |
|
treatment provided by |
Plazi |
|
scientific name |
Pericelis flavomarginata |
| status |
sp. nov. |
Pericelis flavomarginata sp. nov.
[New Japanese name: ĿŦẏǺṷ̕ṷx]
( Figs 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
? Pericelis sp. 6 Ono (2015), p. 71 [the Kerama Islands, Okinawa, Japan]
Etymology. The new specific name flavomarginata (-us, -a, -um) is a compound adjective derived from the Latin flavus and marginatus, meaning “yellow-margined”. It was named after the dorsal surface of the body fringed with a lemon-yellow line.
Material examined. Holotype: ICHUM 6116 View Materials , sagittal sections of the reproductive organs (18 slides), and the rest of the body, unsectioned, preserved in 70% ethanol, collected by D. Uyeno at 10–20 m depth off the coast of Kome-jima (or Yone-jima) (31.4343°N, 130.1212°E), Nomaike , Kagoshima, Japan, July 13, 2019 GoogleMaps . Paratypes: ICHUM 6117 View Materials , sagittal sections (16 slides), collected by Y. Oya at 1–2 m depth of Omura Beach (27.0934°N, 142.1942°E), Chichi-jima Island , the Ogasawara Islands, Japan, September 6, 2016 GoogleMaps ; ICHUM 6118 View Materials , sagittal sections (8 slides), collected by Y. Oya and A. Tsuyuki at 10–15 m depth off the coast of Bonomisaki (31.2541°N, 130.2150°E), Bonotsu , Kagoshima, Japan, July 26, 2018 GoogleMaps ; ICHUM 6119 View Materials , sagittal sections (14 slides), collected by N. Jimi at 12 m depth of Koganezaki Beach (34.8431°N, 138.7625°E), Koganezaki , Shizuoka, Japan, January 26, 2020 GoogleMaps ; ICHUM 6120 View Materials , unsectioned, preserved in 70% ethanol, collected in Shiogaura (31.2547°N, 130.2330°E), Bonotsu , Kagoshima, Japan, July 12, 2019 GoogleMaps ; ICHUM 6121 View Materials , sagittal sections (9 slides), collection data same as holotype GoogleMaps ; ICHUM 6122 View Materials , unsectioned, preserved in 70% ethanol, collected in Shiogaura (31.2547°N, 130.2330°E), Bonotsu , Kagoshima, Japan, July 6, 2019 GoogleMaps .
Type locality. Off the coast of Kome-jima (or Yone-jima) (31.4343°N, 130.1212°E), Nomaike , Kagoshima, Japan GoogleMaps .
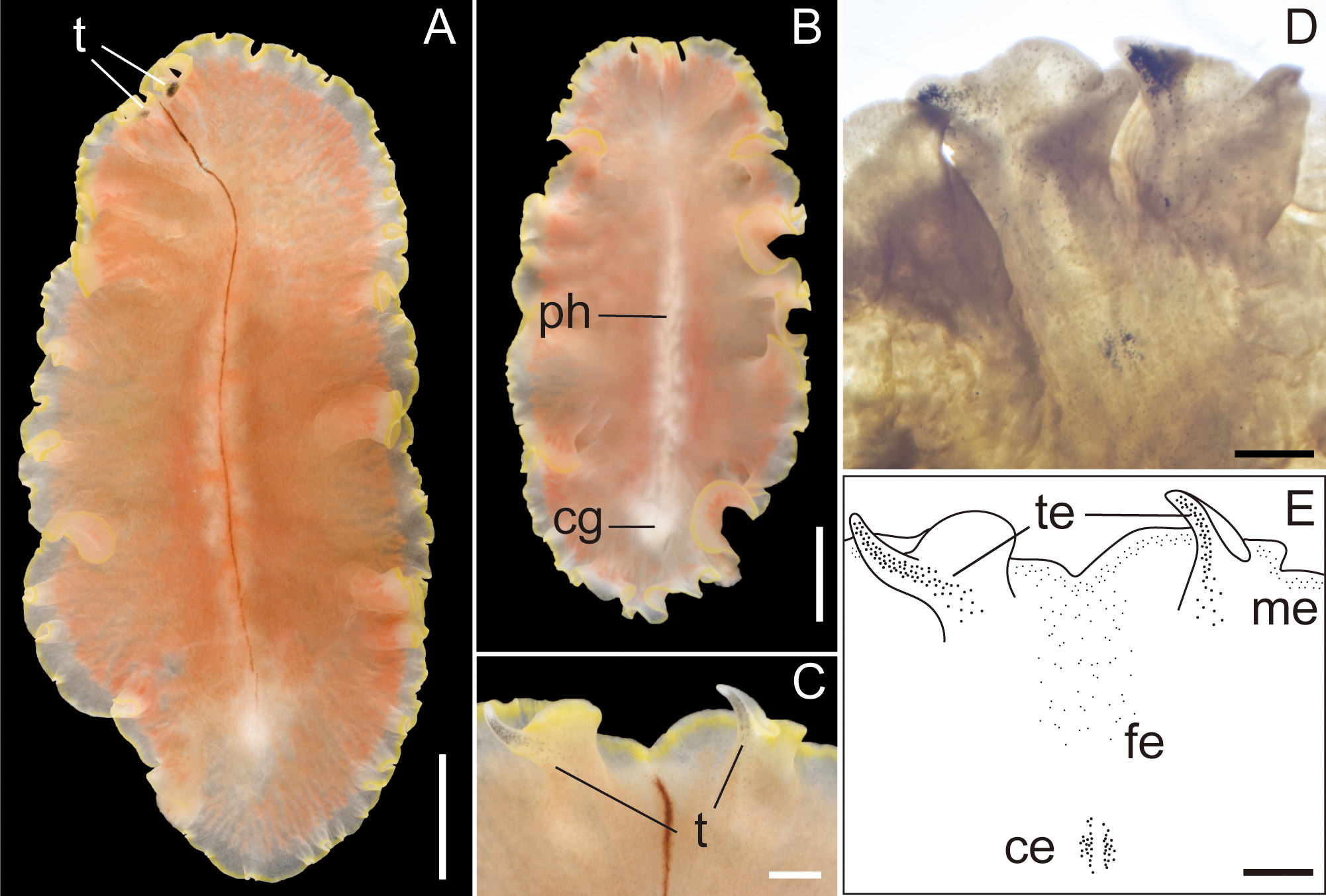
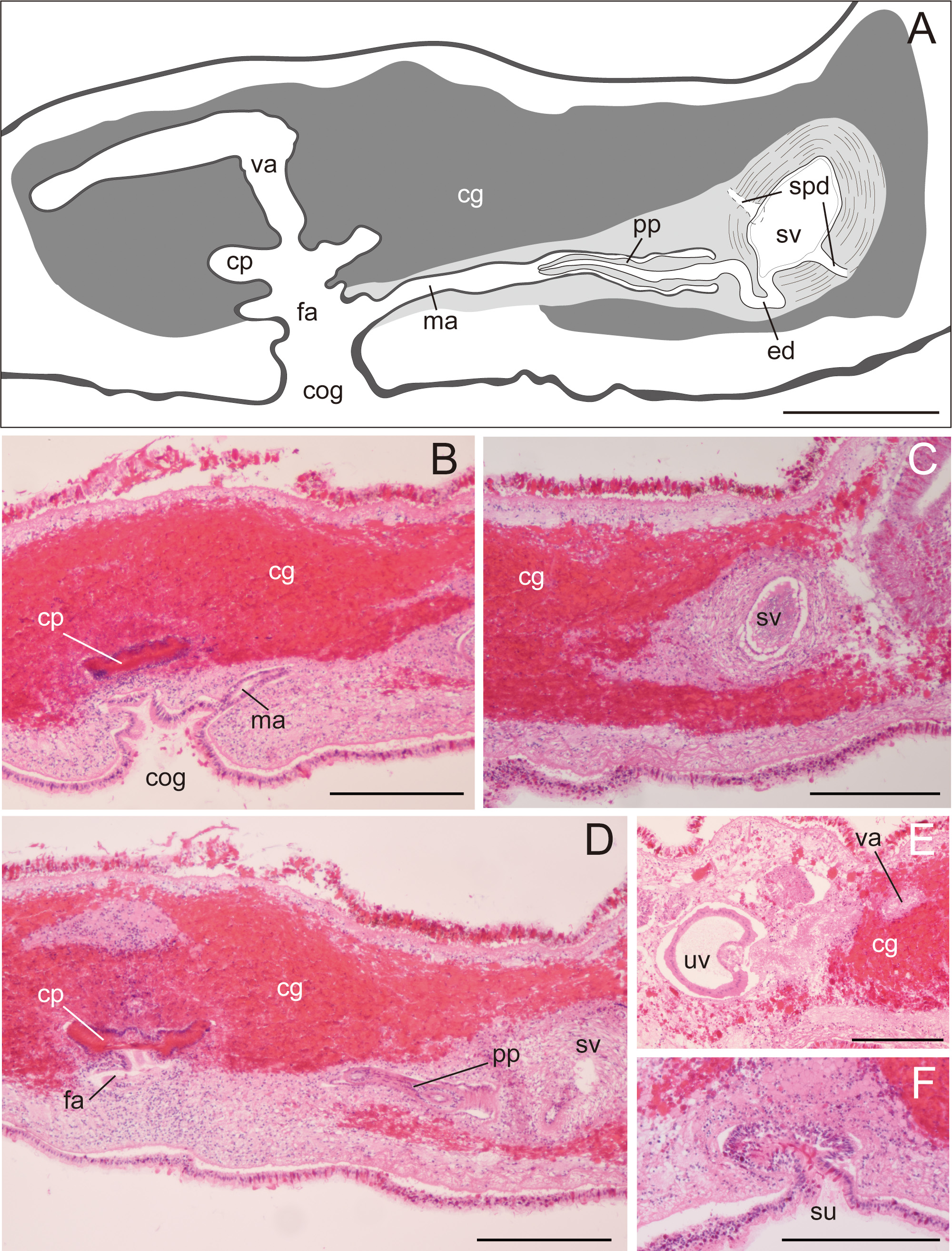
Description. Body elongated oval, slightly tapered posteriorly, 30–48 mm long (32.5 mm in holotype) and 12–35 mm maximum wide (15 mm in holotype) in living state ( Fig. 2A, B View FIGURE 2 ); body margin slightly ruffled. Pair of marginal tentacles apparent; tip of tentacles extending and tapering ( Fig. 2C View FIGURE 2 ). Dorsal surface smooth, translucent, fringed with lemon-yellow line except for translucent tip of marginal tentacle; narrow brown midline extending from anterior edge of body to posterior end of pharynx. Ventral surface translucent, without color pattern. Cerebral eyespots in two elongated clusters, lateral to middorsal brown band ( Fig. 2D, E View FIGURE 2 ). Frontal eyespots scattered between tentacles ( Fig. 2D, E View FIGURE 2 ). Tentacular eyespots abundant at tip, scattered posteriorly ( Fig. 2D, E View FIGURE 2 ). Marginal eyespots in single band, completely encircling body. Intestine highly branched, not anastomosing. Pharynx ruffled, elongated, half of body length, situated on body center ( Fig. 2B View FIGURE 2 ). Male and female gonopores opening in common behind posterior end of pharynx ( Fig. 3A, B View FIGURE 3 ). Male copulatory apparatus consisting of large seminal vesicle and unarmed penis papilla ( Fig. 3A View FIGURE 3 ). Pair of sperm ducts entering laterally into seminal vesicle at point being close to proximal end of ejaculatory duct. Spermiducal bulb absent. Seminal vesicle oval, coated with thick muscular wall, narrowing posteriorly and opening into ejaculatory duct ( Fig. 3A, C View FIGURE 3 ). Without prostatoid organs or prostatic glands. Ejaculatory duct narrow, curving downward before entering penis papilla ( Fig. 3A View FIGURE 3 ). Penis papilla elongate conical unarmed, protruding into long narrow male atrium; former occupying about half of length of latter; both lined with muscularized epithelium ( Fig. 3A, B, D View FIGURE 3 ). Male and female atriums opening to short common atrium and to broad common gonopore ( Fig. 3A, B View FIGURE 3 ). Female copulatory apparatus behind level of male atrium. Cement glands surrounding female copulatory apparatus and extending anterior up to level of seminal vesicle ( Fig. 3 View FIGURE 3 A–D). Cement pouch apparent ( Fig. 3A, B, D View FIGURE 3 ). Vagina curving posteriorly, leading to pair of oviducts; each oviduct running anteriorly, with 4–5 uterine vesicles; posterior-most being largest, arranged posterior to female gonopore ( Fig. 3E View FIGURE 3 ). Lang’s vesicle absent. Sucker well developed, situated in posterior to female copulatory apparatus, near distal end of body ( Fig. 3F View FIGURE 3 ).
Distribution. Our materials were distributed in Koganezaki (Shizuoka), Nomaike and Bonotsu (Kagoshima), Chichi-jima Island (Ogasawara, Tokyo). A similar-looking specimen has been confirmed from the Kerama Islands (Okinawa) ( Ono 2015).
Habitat. So far confirmed under rocks in the intertidal and subtidal zones down to 20 m depth.
Diagnosis. Body elongated oval; body margin slightly ruffled; pair of marginal tentacles with tips extending and tapering; dorsal surface translucent, fringed by lemon-yellow line except for tip of tentacles; narrow brown midline running from anterior edge of body to posterior end of pharynx; pair of cerebral-eyespot clusters anteroposteriorly elongated along brown midline; male and female atriums opening to short common atrium and single gonopore; elongate penis papilla half as long as narrow male atrium; 4–5 uterine vesicles in each oviduct.
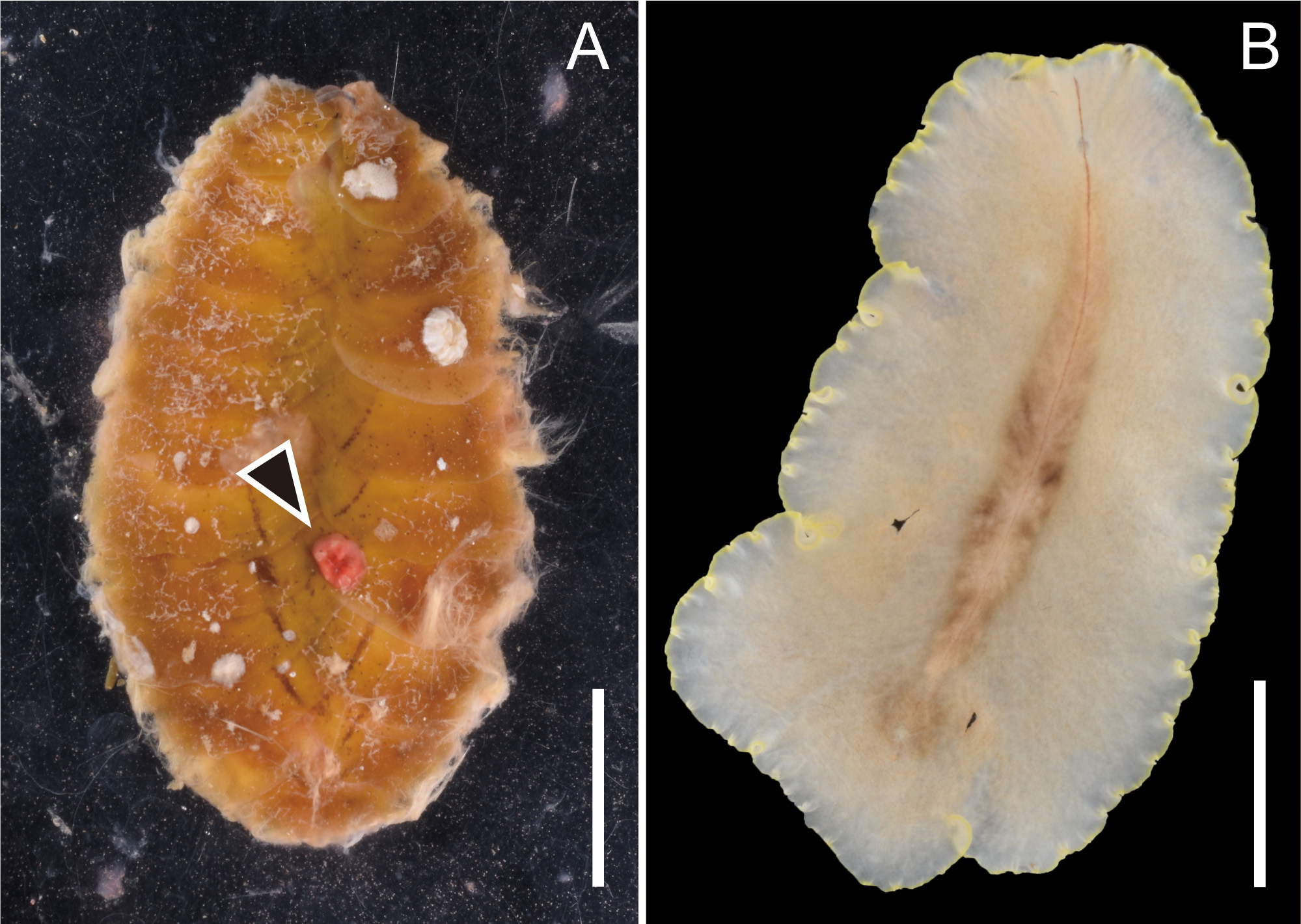
Feeding behavior. We observed P. flavomarginata sp. nov. prey on the scaleworm Iphione muricata . While one individual each of the two species was put together in a plastic bag, we noticed that the flatworm firmly attached to the dorsal side of the scale worm, with its expanded pharynx almost engulfing the prey’s entire body. After violently wiggling, the scaleworm gradually became inactive. We interpreted this behavior as an attempt to escape the polyclad attack. While the scaleworm was still active, the flatworm started sucking out of the scaleworm’s internal tissue. After 1–2 h since we noticed that the flatworm mounted on the scaleworm, the former left the carcass of the latter. Doing the whole process, we were able to observe how the flatworm’s intestinal branches gradually became reddish. After the flatworm left the prey behind, we observed a reddish wound on the dorsal surface of the dead scaleworm ( Fig. 4A View FIGURE 4 ). We compared the freshly fed exemplar with a starving one, and we noticed that the reddish intestinal contents made the gut branches of the polyclad worm more visible ( Figs 2A View FIGURE 2 , 4B View FIGURE 4 ).
Sequences. Partial COI (712 bp) and 28S rDNA sequences from four individuals: LC568540 View Materials (COI) , LC568536 View Materials (28S rDNA, 1008 bp) from ICHUM 6116 View Materials (holotype) ; LC568538 View Materials (COI) , LC568535 View Materials (28S rDNA, 2117 bp) from ICHUM 6117 View Materials (paratype) ; LC568539 View Materials (COI) from ICHUM 6118 View Materials (paratype) ; LC568541 View Materials (COI) , LC568537 View Materials (28S rDNA, 1008 bp) from ICHUM 6119 View Materials (paratype) . There were six variable sites in 712-bp COI sequences; the translated protein sequences were identical. The overlapping 1008 bp of 28S rDNA sequences from the three specimens were identical.
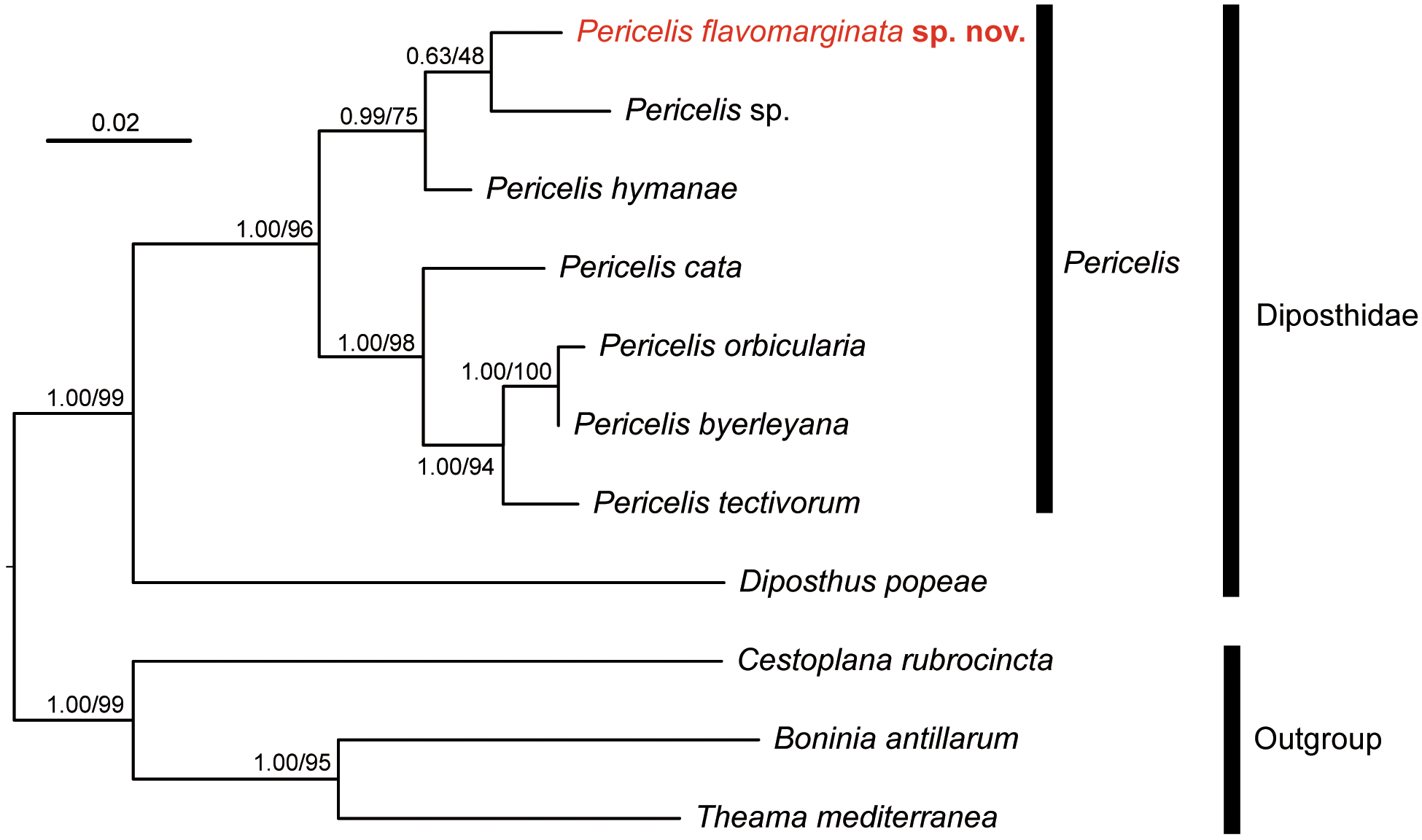
Molecular phylogeny. In the phylogenetic tree, P. flavomarginata sp. nov. was nested with other Pericelis species ( Fig. 5 View FIGURE 5 ). In addition, this species was more closely related to P. hymanae and Pericelis sp. (GenBank MK299354 View Materials ) than to the other four species of Pericelis included in the analysis.
Remarks. Our specimens belong to Pericelis because they conform to the generic diagnosis by having i) a pair of folded marginal tentacles with eyespots, ii) marginal eyespots that encircle the body margin, iii) a long, ruffled pharynx situated centrally, and iv) a male copulatory apparatus without distinct prostatic organs ( Ramos-Sánchez et al. 2020). Pericelis flavomarginata sp. nov. is easily distinguished from P. byerleyana , P. cata , P. nazahui , P. orbicularis , P. sigmeri , and P. tectivorum by their brown coloration ( Dittmann et al. 2019; Ramos-Sánchez et al. 2020), which is in contrast to the lemon-yellow marginal rim and the dorsal narrow brown midline of P. flavomarginata sp. nov. Our new species is similar to P. hymanae in that both species have a narrow brown midline in the dorsal surface of the body. However, P. flavomarginata sp. nov. can be distinguished from P. hymanae by the dorsal marginal coloration (lemon yellow in P. flavomarginata sp. nov. vs. translucent in P. hymanae ). In addition, our new species is distinguishable from P. hymanae by the presence of common gonopore; according to Meixner (1907) and Du Bois-Reymond Marcus & Marcus (1968), the interpretation of whether or not the male and female gonopores are separated may depend on the fixation state. Therefore, this feature should be reexamined based on specimens with or without careful anesthetization.
Pericelis sp. 6 in the Kerama Islands, Okinawa ( Ono 2015) may be identified as P. flavomarginata sp. nov. in terms of its similar dorsal coloration. However, more examination is required with observation of internal morphology and molecular analyses.
The phylogenetic closeness among Pericelis species may reflect similarity in dorsal coloration. In the resulting tree, the seven species of Pericelis were gathered in two clades; the first consists of four species, P. byerleyana , P. cata , P. orbicularis , and P. tectivorum , and the second of three species, P. flavomarginata sp. nov., P. hymanae , and Pericelis sp. ( Fig. 5 View FIGURE 5 ). The constituents of each clade share a similar dorsal color pattern: P. byerleyana , P.cata , P. orbicularis , and P. tectivorum are characterized by cream or beige coloration with a reticulated brown pattern ( Dittmann et al. 2019) whereas P. flavomarginata sp. nov. and P. hymanae share a brown midline on translucent background ( Poulter 1974). We predict that the unidentified Pericelis sp. of Cuadrado et al. (unpubl.) from S„o Vincente ( Cape Verde) would also display a colored dorsal midline on a uniform background, although no information is currently available to us as to its coloration and morphology. Also, the phylogenetic positions of P. nazahui and P. sigmeri , which have brown coloration, should be revealed in future studies.
Our study is the first report of a polyclad flatworm feeding on a scaleworm, although some more experimentation is needed to evaluate if P. flavomarginata sp. nov. displays the same diet preferences in its habitat. Among the polyclad species with the ruffled pharynx, predatory behavior and mechanisms have been observed and proposed for several species. Some feed on ascidians, crustaceans, and gastropods, with their pharyngeal glandular epithelium secreting enzymes that help predigesting the prey’s tissue before sucking them ( Prudhoe 1985; Newman & Cannon 2003; Jie et al. 2013). Pericelis flavomarginata sp. nov. seemed to feed on the scaleworm I. muricata in a similar way. In our observation, the polyclad flatworm held firmly the dorsal body of I. muricata with its expanded pharynx and appeared to suck the digested internal tissue of the prey through dorsal wound ( Fig. 4A View FIGURE 4 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Cotylea |
|
Family |
|
|
Genus |