Ctenocephalides felis felis (Bouché, 1835), felis (Bouche, 1835
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4374.1.8 |
|
publication LSID |
lsid:zoobank.org:pub:F59FE90F-C936-44A8-8F69-E1DEAA08CF7B |
|
DOI |
https://doi.org/10.5281/zenodo.5970137 |
|
persistent identifier |
https://treatment.plazi.org/id/03E187D1-FFB0-FFED-FF4E-FA51FBF4FC0A |
|
treatment provided by |
Plazi |
|
scientific name |
Ctenocephalides felis felis (Bouché, 1835) |
| status |
|
Ctenocephalides felis felis (Bouché, 1835) View in CoL
Type host and locality. “Housecat”; Type locality not indicated.
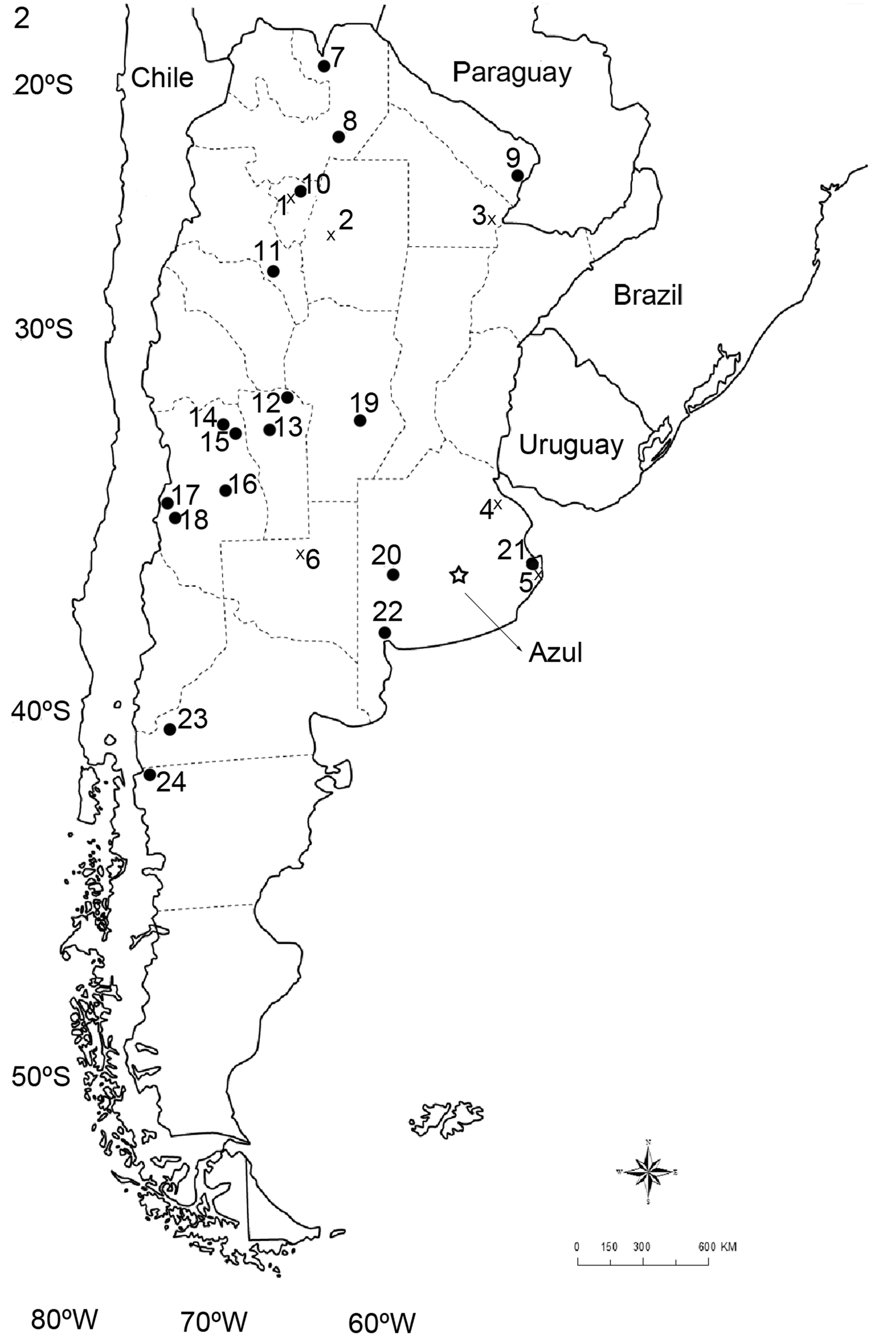
Geographic range. Cosmopolitan. In Argentina the distribution probably includes all the country; however, these are the published records ( Fig. 2 View FIGURE 2 ): Chaco, La Pampa, La Rioja, Santiago del Estero, and Tucumán. In Buenos Aires province: Los Yngleses and Ajó (General Lavalle) ( Lareschi et al. 2016).
Other known hosts for Argentina. Mammalia-Artiodactyla-Cervidae— M. americana , M. nana . Didelphimorphia- Didelphidae— D. albiventris , Lutreolina crassicaudata , Didelphis sp. Carnivora-Canidae— C. familiaris , L. griseus , L. gymnocercus . Felidae— Felis catus, P. concolor . Procyonidae— N. nasua . Rodentia —Cricetidae— G. griseoflavus . Muridae— Rattus sp. Primates—Hominidae- Homo sapiens ( Lareschi et al. 2016) .
Specimens examined. Azul , Buenos Aires province, 13 females ( MLP JS1 View Materials _3, MLP JS1 View Materials _4, MLP JS1 View Materials _5, MLP JS3 View Materials _3, MLP JS6 View Materials _2, MLP JS7 View Materials _2, MLP JS7 View Materials _3, MLP JS10 View Materials _2, MLP JS10 View Materials _3, MLP JS13 View Materials _3, MLP JS14 View Materials _2, MLP JS15 View Materials _1, MLP JS15 View Materials _3) ; 8 males (MLP JS1_6, MLP JS1_7, MLP JS6_3, MLP JS6_4, MLP JS7_4, MLP JS7_5, MLP JS13_4, MLP JS15_2).
parasitological indexes. n(fleas anterior-right flank) = 21; n(pigs) = 30; n(parasitized pigs) = 8; prevalence (anterior-right flank) = 27%; Mean abundance (anterior-right flank) = 0.7; mean intensity (anterior-right flank)= 2.6
Remarks. Ctenocephalides felis felis is characterized by the following morphological characters: Head longer; frons oblique and strongly curved. Genal comb horizontal, with 8 spines each side, first spine of genal comb about same length as second. Eye large, dark. Occiput bearing 1 long bristle above middle of antennal fossa. Labial palpus with five segments; anterior margin thickened, well sclerotized. Fore tarsal segment V of male with only 2 sub-apical spiniform setae. Metepisternum with 1 or 2 long setae. Male with sternite VIII enlarged, enclosing most of tail end posteriorly. Fixed process of clasper with divided apical portion. Movable process of clasper arising from fixed process proper. Manubrium of clasper little dilated apically. Female without small setae above antennal fossa. Spermatheca with subglobular bulga and hila longer and with end curved.
The cat flea, C. felis felis , is extremely common on cats and dogs in many temperate and tropical regions, but it also infests rats and other wildlife. It represents the great majority of fleas in human homes ( Durden et al. 2005; Bitam et al. 2010). This flea is a recognized vector of Rickettsia felis in several countries, including Argentina ( Brown & Macaluso 2016; Nava et al. 2008). Infection with this agent in humans produces a disease known as flea-borne spotted fever (or cat flea typhus). This disease is considered an emergent global threat to human health, with cases likely underestimated due to similarities in clinical signs with other febrile illnesses (e.g. fever, rash, headache, and myalgia) and limited access to appropriate laboratory tests ( Brown & Macaluso 2016). Moreover, this flea species can be naturally infected with Rickettsia typhi , the causative agent of murine typhus, a zoonotic disease that circulates in rodents via the oriental rat flea. It is also a vector of Bartonella henselae , the causative agent of Cat scratch disease ( Bitam et al. 2010).
This study represents the first records of C. felis felis and P. irritans parasitizing S. scrofa in Argentina. From an epidemiological point of view C. felis felis and P. irritans are vectors of several pathogens in various regions of the world, including Argentina ( Nava et al. 2008; Bitam et al. 2010). Both species of fleas are considered synanthropic and cosmopolitan, being able to disperse pathogens to new regions and hosts ( Bitam et al. 2010). As noted by several authors, despite their cosmopolitanism, distribution of these fleas is not uniform. Instead, they are distributed in patches characterized by host and environmental conditions that are favourable for each given species (see Traub 1980; Beaucournu & Menier 1998; Beaucournu & Pascal 1998 in Krasnov 2008). Our results agree with these authors, because considering that the management conditions (e.g. infrastructure, sanitary controls) of all farms examined in this study were similar, fleas were found only in farms in central Buenos Aires province (Azul locality); in the north of the province these parasites were not found. Parasitological index values obtained in this study (tested only for an area of the host's body, see Materials and Methods) suggest that the presence of both flea species in Azul is not accidental. These results contribute to the knowledge of the biology of these fleas whose presence could respond to environmental characteristics. In most countries of the world there is constant concern over flea control, although the incidence of flea-borne diseases is much greater than is generally recognized by physicians and health authorities. Fleas, as hosts for a wide range of largely understudied pathogens, are no exception, and flea-borne diseases may re-emerge in epidemic form. Examples of this are the changing ecology of murine typhus, the finding of Rickettsia spp. in new hosts, and the finding of fleas on new hosts or in geographical areas previously unreported in the literature. The past decades have seen a dramatic change in geographic and host ranges of many vector-borne pathogens and the diseases they cause. This process is often driven by climate change and the destruction of wild habitats due to human behaviour modifications ( Bitam et al. 2010). Agricultural and livestock development is one of the factors of emergence and re-emergence of diseases, as a common pathway in altering the environment ( Jones et al. 2013).
Considering that in the studied region in this work a strong swine livestock activity is carried out, with a high frequency of contact between pigs and humans, the presence of two fleas with sanitary importance in domestic pigs involves a potential risk to humans to acquire pathogens. Also, these findings represent a risk factor for humans for other two main reasons: 1) in pig farms of Argentina rodents (mainly rats and mice) are very common ( Lovera et al. 2015), and are recognized reservoirs of several of the zoonotic pathogens transmitted by fleas ( Linardi & Guimarães 2000; Bitam et al. 2010); 2) there is strong interaction between domestic and wild pigs in the province of Buenos Aires, presenting an opportunity for pathogens, previously eradicated from domestic populations of pigs, to be re-introduced into those specific pathogen-free populations ( Cooper et al. 2010). Feral pig infectious disease research is focused on the threat that feral pigs could contaminate clean domestic herds and is based on the previously known parasites and pathogens of domestic pigs and their long established disease cycles. Some bacterial and parasitic pathogens of feral pigs that can be potentially transmitted to humans and cause disease are bubonic plague and tularemia, and emerging viral diseases of high impact on human health, such as influenza and Hepatitis E (HEV) ( Graves 1984; Mullen & Durden 2009). A few epidemiological studies of feral pigs conducted in Argentina have detected the transmission of hemoparasites as Babesia spp., Anaplasma spp., and Mycoplasma suis , whose vectors could be haematophagous ectoparasites, to domestic pigs ( Scioscia et al. 2011).
Our results expand knowledge about the flea fauna associated with S. scrofa , and enable us to know more about the distribution, biology and ecology of P. irritans and C. felis felis . This information is relevant for smallholder farmers working on pigs and also for people engaged in all aspects of public health surveillance so they are aware of the distribution of these two flea species and prepared to control them when necessary.
| MLP |
Museo de La Plata |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |