Phalloceros, Eigenmann, 1907
|
publication ID |
https://doi.org/ 10.1093/zoolinnean/zlad034 |
|
DOI |
https://doi.org/10.5281/zenodo.8328981 |
|
persistent identifier |
https://treatment.plazi.org/id/03E187BC-B06C-FFE6-20FD-1155FDBCFA43 |
|
treatment provided by |
Plazi |
|
scientific name |
Phalloceros |
| status |
|
Low genetic diversity in Phalloceros View in CoL View at ENA species?
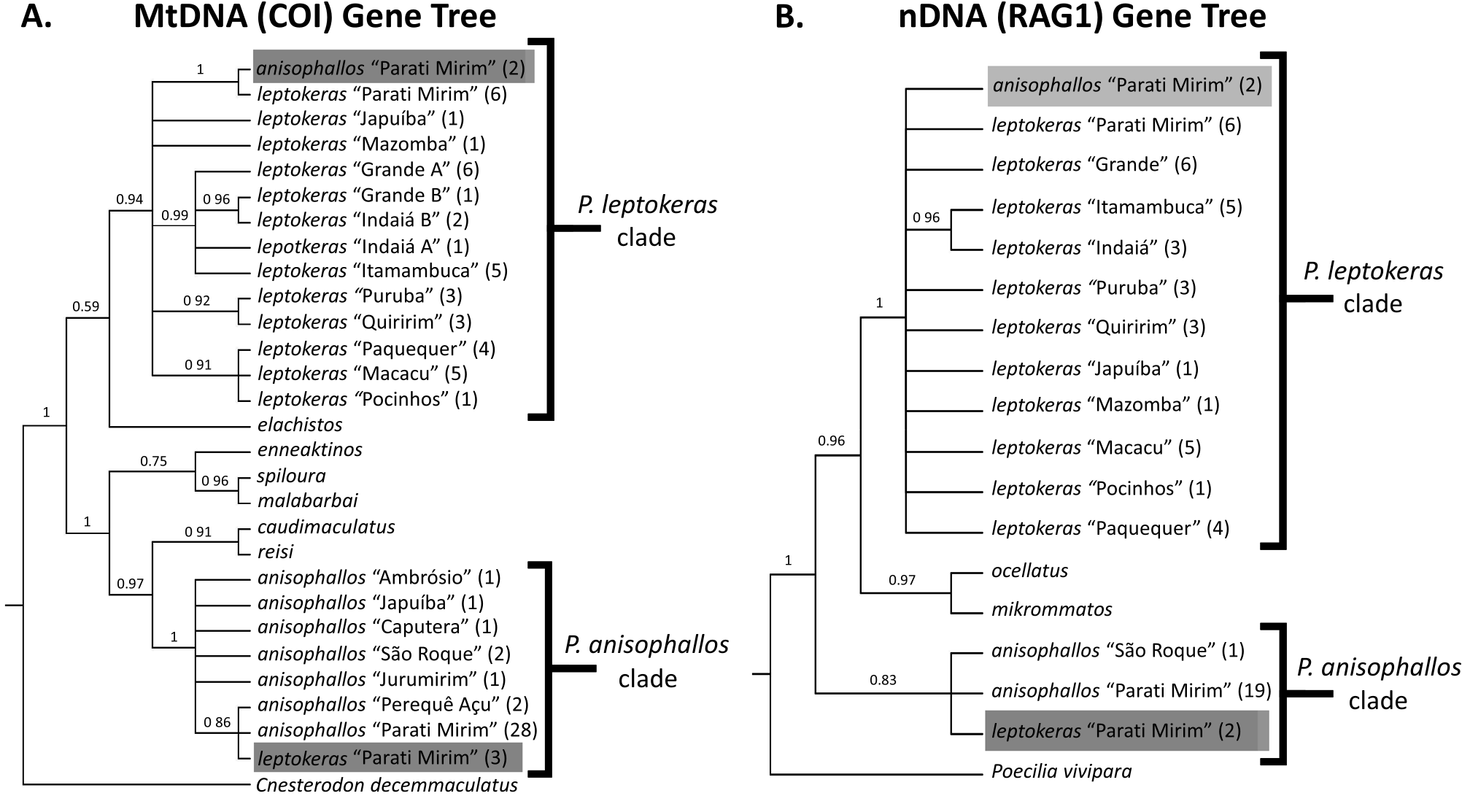
Another noteworthy finding from our molecular results concerns the low genetic diversity of the populations we studied. Despite having obtained mtDNA sequences from 31 individuals of P. anisophallos and eight of P. leptokeras from the Parati-Mirim River, all sampled individuals within each of these species had the same haplotype sequence ( Fig. 2 View Figure 2 ). A perusal of several other drainages that had multiple individuals sampled for the mtDNA gene showed the same pattern (i.e. Itamambuca, Puruba, Quiririm, Paquequer, Macacu and Perequê Açu Rivers; Fig. 2A View Figure 2 ). Only the Grande and Indaiá Rivers showed more than one distinctive haplotype sequence ( Souto-Santos et al. 2023). These occurrences might have been attributable to gene flow that occurred when the sea level was low enough to join these adjacent rivers into one or they might represent ancestral polymorphisms that originated in the ancestral population for the two rivers. However, the low genetic diversity seen in each of these isolated populations could have arisen via bottlenecks or founder events.
Problems with identifying species of Phalloceros
We also found that intraspecific variation in morphology in these species can lead to incorrect species IDs, especially with immature individuals.This latter finding is of importance to researchers who study Phalloceros because it shows that morphology alone might be insufficient to identify individuals in systems with multiple co-occurring species of Phalloceros . However, our results show that standard mtDNA barcode sequences and our newly developed nDNA barcode marker provide reliable species IDs for Phalloceros species.
Of the 30 adult (25 female and five male) specimens in our study, only one (MNTI 14559) was misidentified using genital characters. This female individual was misidentified initially as P. anisophallos because it had a right-turned papilla. However, our re-examination of this specimen revealed that the papilla on this individual was aberrant; instead of pointing distally, as is the usual case for P. anisophallos , it was pointed in a latero-anterior direction like the normal condition for mature P. leptokeras females. Moreover, the papilla had a fleshy process protruding outwards from its anterior side, which is a unique characteristic of P. leptokeras ( Souto-Santos et al. 2023) . Although aberrantly shaped papillae have been reported for P. harpagos , there is negligible intraspecific variation in this trait or in the shapes of species-specific gonopodia ( Lucinda 2008; Ono and Shibatta 2015; Thomaz et al. 2019). Thus, our study supports the practice of using genitalia to identify adult individuals of P. leptokeras and P. anisophallos .
Although our results showed that adult Phalloceros individuals from the Parati-Mirim River can be identified reliably as P. anisophallos or P. leptokeras based on the genital traits, we could not use these morphological features to identify the immature individuals in our sample because their genitals were either not visible (in juveniles) or they were underdeveloped (in subadults). We therefore identified these individuals provisionally using the presence or absence of dark lateral spots, following Lucinda (2008). However, substantial intraspecific variation in this trait explains the poor correlation between the presence of spots and identities of Phalloceros species in the Parati-Mirim River. This practice led us to misidentify four of the nine immature individuals in our sample. Most surprising of all was that 72% of our P. anisophallos specimens lacked spots, which contradicts Lucinda’s (2008) assertion that the presence of lateral spots is a diagnostic character for this species. Although we also discovered that individuals of P. leptokeras from the Parati-Mirim River departed from the expected no-spot pattern ( Lucinda 2008), our observations that individuals of this species can show variable spotting patterns (see Table 1 View Table 1 ) confirms the earlier findings of Souto-Santos et al. (2023). Thus, an important implication of our study for Phalloceros researchers is that immature individuals of these two species from the Parati-Mirim River can be identified only using molecular barcode sequences.
How prevalent is syntopy among species of Phalloceros ?
Lucinda (2008: p. 156) noted that in Phalloceros , ‘some species are sympatric or even syntopic’, and then listed the pairs P. caudimaculatus – P. heptaktinos and P. harpagos – P. leptokeras as syntopic pairs of Phalloceros . However, Thomaz et al. (2019: p. 270) pointed out that P. caudimaculatus and P. heptaktinos occur in different portions of the same river basin and thus might not be syntopic. Indeed, our re-examination of Lucinda’s data suggests that only the P. harpagos – P. leptokeras pair represents a valid case of syntopy in his argument. Thomaz et al. (2019: p. 270) performed a genus-wide analysis of species distributions for Phallocero s and concluded that ≥ 14 of the 22 then-known species can be found in sympatry with one or more congeners and that most of the sympatric cases represented instances of syntopy. However, our re-analysis of their data shows that Thomaz et al. (2019) had uncovered only four valid cases of syntopic species: P. alessandre – P. pellos , P. malabarbai – P. spiloura , P. megapolos – P. pellos and P. megapolos – P. titthos (Supporting Information, Table S3). Nonetheless, the new data we have presented here (Supporting Information, Table S3) suggest that syntopy is not only widespread in the genus, but that ≥ 14 species of Phalloceros occur in syntopy with a congener, thereby supporting the conjecture of Thomaz et al. (2019). We also found that every case of syntopy involved only two species. Moreover, when we examined these syntopic pairs in light of their likely topological placements in the Phalloceros species tree ( Thomaz et al. 2019: fig. 3), all these syntopic pairs consisted of non-sister species, similar to the finding reported by Thomaz et al. (2019), although, again, their conclusion was based mainly on records of sympatry and not syntopy.
The independent dataset we obtained for this study also strongly corroborates the conjecture of Thomaz et al. (2019) concerning the prevalence of mismatched genitalia in syntopic Phalloceros . Indeed, all new cases of syntopy that we identified involved two coexisting species of Phalloceros had genitalia mismatched to some degree (Supporting Information, Table S3). This study thus confirms that syntopy is widespread in Phalloceros and that these species pairs seem always to consist of species with apparently mismatched genitals. However, if Lucinda’s (2008) claim that P. caudimaculatus and P. heptaktinos can be found in syntopy is eventually confirmed, then this species pair would represent an intriguing exception to the syntopy/mismatched genitalia rule in Phallocero s. This is because the mechanical isolation hypothesis implies that co-occurring species of Phalloceros having ‘matched’ genital morphologies can interbreed with each other. The existence of such an exceptional case would thus be problematic for this hypothesis unless a future study can show that these two species are interbreeding with each other.
Do all mismatches in genitalia cause reproductive isolation?
Our results are consistent with the hypothesis that syntopic species of Phalloceros with mismatched genitalia are unable to hybridize with each other. However, even if this is true for the P. anisophallos and P. leptokeras populations we studied, this might not necessarily apply to other syntopic pairs of Phalloceros , because there are at least five different types of ‘mismatched genitalia’ ( Table 2 View Table 2 ), which could vary in their efficacies to inhibit cross-species fertilization. Of the three genital characters that show variation at the interspecific level, divergent papillae (with or without accompanying divergences in hook number and appendix shape) characterize 11 of 17 syntopic species pairs ( Table 2 View Table 2 ), hence orientation of the papillae might be a primary determinant of whether two co-occurring species of Phalloceros can interbreed with each other. Interestingly, the remaining six syntopic pairs showed interspecific differences in only hook number (four syntopic pairs) and hook number + shape of appendices (two pairs; Table 2 View Table 2 ). Thus, there might be other factors involved (e.g. species-specific courtship behaviours) that help to maintain the species boundaries in these six exceptional species pairs, or perhaps they interbreed with each other.
The Phalloceros clade is an exciting study system in which to explore the evolutionary significance of mismatched genitalia to speciation and the subsequent maintenance of species boundaries. Although we presented evidence that is consistent with the mechanical isolation hypothesis for Phalloceros , our findings suffer from two important limitations. First, we examined hybridization in only a small number of individuals from each population using two molecular markers. Had we sampled a larger number of individuals and/or genomic loci, we might have obtained evidence showing that P. anisophallos and P. leptokeras in the Parati-Mirim River have interbred with each other. Second, our findings, and all relevant published works on Phalloceros , have presented only correlational evidence in support of this hypothesis. In the future, researchers should explore the two promising avenues of population genomics and laboratory breeding experiments, which might shed much more light on the functional and evolutionary significance of mismatched genitalia in these fishes. The former approach would allow researchers to determine the levels, if any, of historical gene flow between co-occurring Phalloceros species that exemplify each of the observed mismatch variants listed in Table 2 View Table 2 , and between coexisting species that have matched genitalia. In the latter approach, researchers should conduct laboratory breeding experiments between different species of Phalloceros to determine whether mismatched genitalia can inhibit cross-species fertilization. If the results confirm that mismatched genitalia do act as mechanical barriers to fertilization, then further experiments can be done to ascertain the relative efficacies of the different mismatch possibilities and to test the hypothesis that co-occurring species having matched genitalia are able to interbreed. Only after these studies are completed will we be able to understand fully the evolutionary significance of genital morphology variation in Phalloceros and its possible link to their diversification. This, in turn, might reveal the general importance of mismatched genitalia as a mechanism for reproductive isolation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.