Muelleroecia, 2011
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2857.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03DC87FD-EC04-FFCD-6FDD-F8D1A493FD64 |
|
treatment provided by |
Felipe |
|
scientific name |
Muelleroecia |
| status |
gen. nov. |
MUELLEROECIA Chavtur & Angel View in CoL new genus
Synonymy.
1906a Conchoecia rotundata group—Müller, p. 79 (part).
1949 Metaconchoecia — Granata & Caporiacco, p 15 (nomen nudum).
1953 Conchoecia —Iles, p 269 (part).
1962 Conchoecia —Rudjakov, p. 186 (part).
1968 C onchoecia —Deevey, p. 57 (part).
1973 Metaconchoecia —Poulsen, p. 70 (part).
1974 Conchoecia —Deevey, p. 362 (part).
1977a Conchoecia —Chavtur, p 146 (part).
1980 Conchoecia rotundata group—Deevey and Brooks, p. 85 (part).
1982a Conchoecia —Deevey, p. 148 (part).
1981 Conchoecia skogsbergi species complex—Gooday, p. 141 (part).
1986 Metaconchoecia Granata & Caporiacco —Kempf, p. 498 (part).
1993 Conchoecia (= Metaconchoecia )—Angel, p. 158.
1999 Metaconchoecia —Angel, Figs.9.58 View FIGURE 9 –76 (part).
Etymology. The name is derived from “Mueller” in honour of G.W Müller the pioneer of halocyprid taxonomy whose contribution overshadows us all, and “-oecia” from the Greek word “οƖκοσ” meaning house, from which the terms ecology and economy have been derived; this ending that has become standard for the genera of Conchoeciinae .
Type species: Conchoecia glandulosa ( Müller 1906a) View in CoL .
Composition: This genus includes at least two species, and possibly four further putative species:
Conchoecia glandulosa ( Müller 1906a) View in CoL
Conchoecia macromma ( Müller 1906a)
M. sp 1. aff. C. macromma ( Muller 1906a) sensu Deevey 1974
M. sp 2. aff. C. macromma ( Muller 1906a) sensu Deevey 1982a
M. sp 3. aff. C. macromma ( Müller 1906a) sensu Poulsen 1973
M, sp 4. aff. C. macromma ( Müller 1906a) sensu Angel 1981b
Differential diagnosis. Metaconchoeciini species with elongate, tapering carapaces 0.84–1.95 mm in length. The LAG opens on the dorsal surface 20–35%CL posterior to the tip of the rostrum. The RAG opens at the apex of an angle on the posterior margin about 15%CL below the PDC. The male A1 e-seta has an armature of 13–15 slim spines.
Description. Males. Carapace. The length range is 0.84–1.95 mm. The carapace is elongate. The maximum height (43–50%CL) is posterior to midlength, so the carapace tapers anteriorly. The LAG opens on the dorsal surface 20–35%CL behind the tip of the rostrum and 13.5–17%CL behind the anterior end of the hinge. The RAG opens 14.5–15.5%CL below the PDC (i.e. 24–28% of the carapace height). The rostrum is relatively well developed, and is 6–11%CL. There is no surface ornamentation.
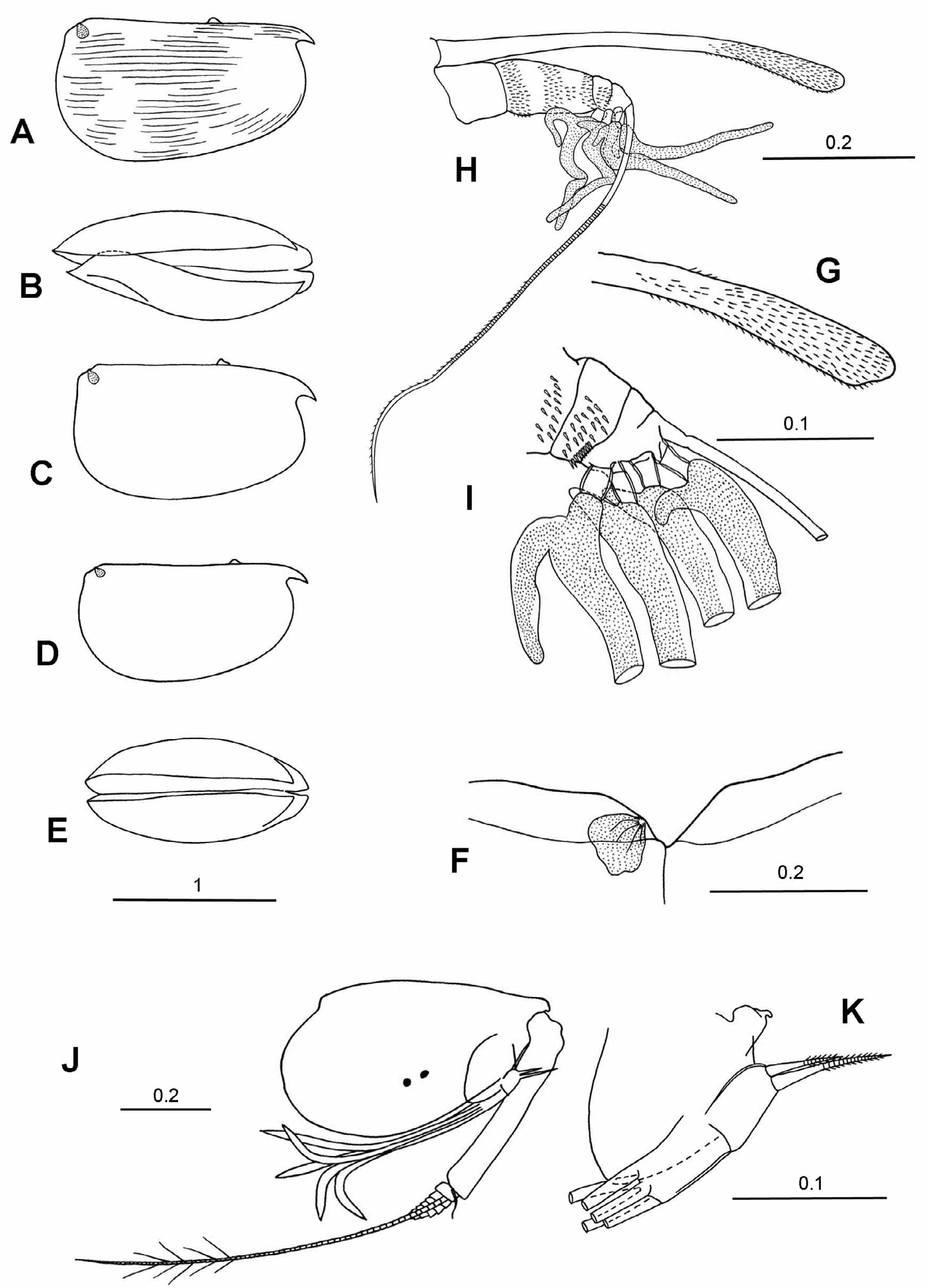
Frontal organ. The capitulum is half the length of the shaft. Even so, it is comparatively long (15–20%CL) and slim (little broader than the shaft). It is either straight or slightly bowed with a rounded end. The shaft is segmented with an articulation just posterior to where the retaining seta from the second segment of first antenna wraps around the shaft. The whole frontal organ extends well beyond the end of the A1.
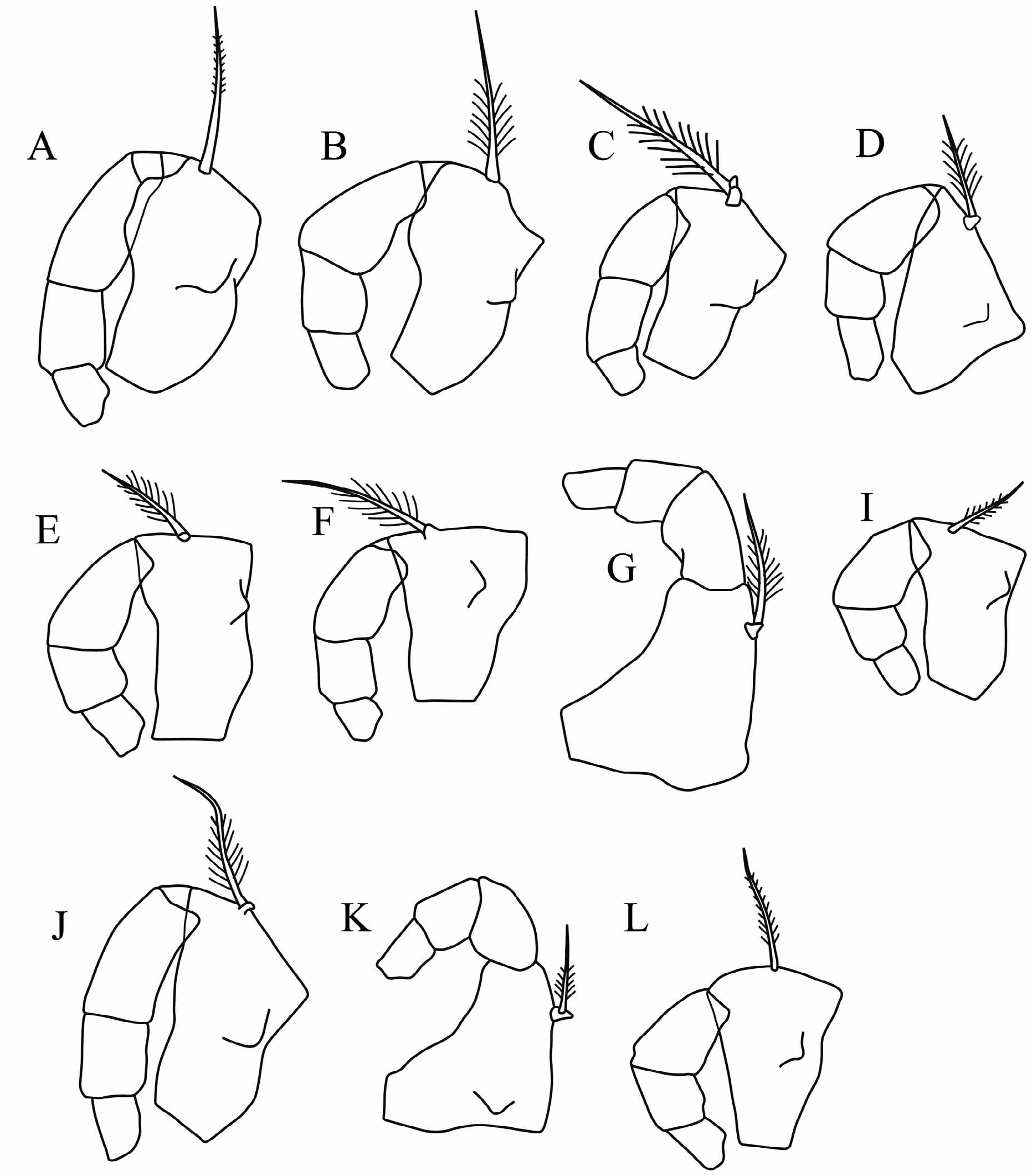
First antenna. The limb segmentation is well defined, and the first segment is slightly shorter than the second. Overall, the length of the limb is 33.5–37.0%CL and is similar in length to the shaft of the frontal organ. The a-seta is S-shaped and swollen at its base. Otherwise it is simple and long (16–21%CL) and it extends back well beyond the suture between the first and second segments. The c-seta is short (4.0–8.2%CL) and 75–125% of the combined lengths of the third, fourth and fifth segments. The b-seta is slightly shorter than the d-seta, and the e-seta is slightly longer, 44–55%CL (i.e. 1.5x the length of the limb). The armature of the e-seta generally comprises 13–15 pairs (i.e. 26–30) of spines. The spines are usually long enough for the tips of one pair to overlap the bases of the next pair, but in M. sp 4. aff. C. macromma the 14 pairs of spines do not overlap.
Second antenna. The protopodite is less than half the carapace length (46–47%CL). The exopodite is quite slim, and the first segment is 40–50% the length of the protopodite (i.e. 20–24%CL). The g-seta is similar in length to the protopodite (40–48%CL), and the f-seta is 90% of the g-seta. Both these setae are slim with pointed ends. The h-, i- and j-setae are simple, undivided, and subequal (16–17.7%CL). The c- and d-setae are slim and short. The e-seta is minute. The hook appendages on the endopodites are curved with rounded ends, and the left hook is, as usual, smaller and straighter.
Labrum. In common with all the other genera in the tribe, the labrum is deeply cleft and flanked by short stubby filaments.
Mandible ( Fig. 2I View FIGURE 2 ). The exopodite is developed as a process that carries a long, relatively slim seta with long setules. The coxale is relatively short, about half the length of the endopodite. The terminal segment of the endopodite carries the usual seven setae, two of which are developed into long claws. The longest claw is similar in length to the endopodite (20–23%CL).
Fifth limb. The setation of the limb matches the general pattern for the tribe. The middle terminal claw seta is 8%CL; the dorsal and ventral setae are 75–90% and <50% of the length of the middle seta, respectively.
Sixth limb. The terminal setae are subequal (35%CL), and distally all three carry long setules.
Caudal furca. The longest of the eight pairs of caudal spines is 19%CL. There is an unpaired seta, which is 15% the length of the longest caudal spine.
Copulatory appendage. The appendage is about 31–32%CL. It is straight and relatively parallel-sided with a rounded tip. There are 4–5 oblique muscle bands.
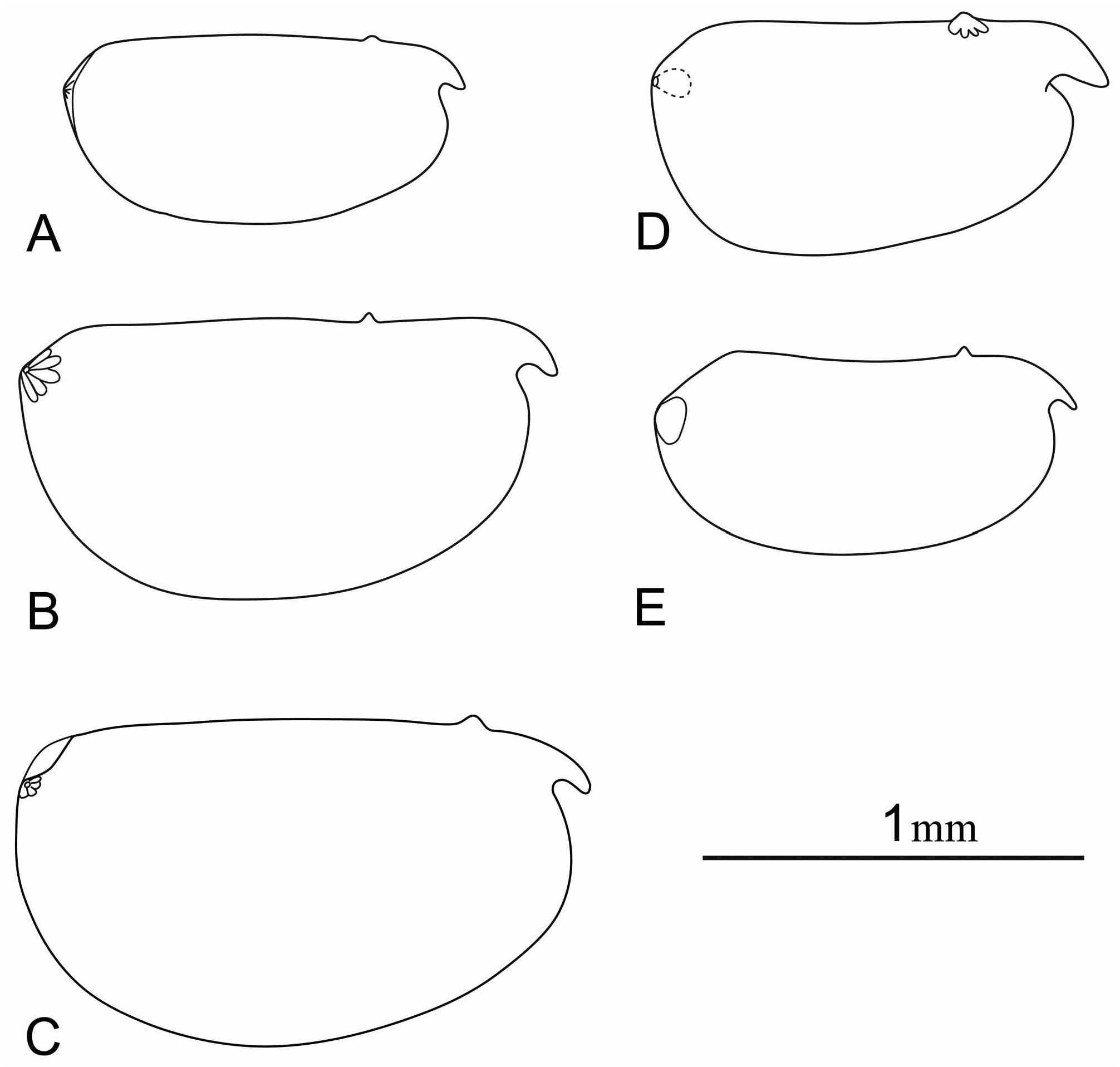
Females. Carapace. The length range is 1.0– 1.95 mm. The carapace is elongate. Its maximum height of 42– 57%CL occurs at or behind midlength. The LAG opens 20–35%CL posterior to the tip of the rostrum and 15– 17%CL posterior to the anterior edge of the hinge. The RAG opens at the apex of an angle on the posterior margin, 17.5–20.5%CL posterior to the posterior end of the hinge and 15.5–20.5%CL below the dorsal margin. The rostrum is relatively well developed (Lrost is 9–12.5%CL), whereas the incisure is somewhat shallower (5–12%CL). There is no surface sculpturing. M. glandulosa is unusual in that in ventral aspect the rostrum is obscured by the anterior bulge of the margin of the carapace below the incisure (see Fig. 5 View FIGURE 5 ), but in all forms of M. macromma the tips of the rostra are clearly visible in ventral aspect.
Frontal organ. The shaft and capitulum are differentiated only by a shallow notch. The shaft (22%CL) is longer than the limb of A1. The capitulum is quite long (7.5–12.5%CL) and is broader than the shaft. It is very slightly down-curved and may have a dorsal indentation near its base. It is inflated distally, and its end is either rounded or slightly pointed. The shaft is either one- or two-segmented (see M. glandulosa, Angel 1993 fig. 53).
First antenna. The basal limb segments are fused, and the limb’s overall length is 15–17%CL. The sensory setae are simple (10–14.3%CL) and less than half the length of the e-seta (27–34.5%CL). Along its trailing edge the e-seta has fine spines distal to the ends of the other setae. In common with the majority of species in the tribe, there is no dorsal seta.
Second antenna. The protopodite is relatively short (36–42%CL). The exopodite is slim and long relative to the protopodite (62–80% of its length). The swimming setae are slightly longer than, or similar in length to, the protopodite. The g-seta on the endopodite is 23–31%CL, and is only a little longer than the other four setae. All these setae are unflattened and have pointed tips.
Labrum, Mandible and Maxilla are similar in structure to those of males.
Fifth limb. The epipodial appendage has three groups of 4 + 4 + 4 setae in M. glandulosa , but there are no data for M. macromma . Ventrally the basale carries a group of three setae at midlength and one or two subterminal seta. Laterally there are two or three setae. Dorsally there is both a long bare seta that extends beyond the end of the limb and a shorter seta with long setules. The first endopodite segment carries two ventral setae and one dorsal seta, all of which extend beyond the end of the segment. The terminal segment carries three terminal setae; the central seta is the longest and is 7.5–8.5%CL. The dorsal and ventral setae are respectively 80% and 60% the length of the central seta.
Sixth limb. The epipodial appendage also has three groups of 6 (or 7) + 5 + 5 setae. The basale carries 4 or 5 ventral setae. Laterally there are up to three setae, and dorsally there is a long subterminal seta and a shorter seta with long setules. The first endopodite segment has a very short ventral seta, but no dorsal seta. The second segment carries a medial dorsal seta and a medial ventral seta, both of which extend almost to the end of the segment. The terminal segment has three unequal setae. The central seta is the longest, and is 15%CL in M. glandulosa , and 10–12%CL in M. sp 4. aff. C. macromma . The dorsal and ventral setae are respectively 80% and 60% of the longest central seta.
Remarks. M. glandulosa is relatively uncommon in collections, but this moderately-sized, deep bathypelagic species occurs regularly whenever samples have been collected from depths> 2000m in the North Atlantic. Like many deep-living species it is fragile and so few specimens have been collected in good condition. It has a large size range. Females collected from the North and Equatorial Atlantic range 1.30–1.74 mm (mean 1.46 mm, n = 29), and males range 1.36–1.80 mm (mean 1.52 mm, n = 11). These are unusually broad size ranges, casting doubt on whether all these specimens are the same species. Similarly, Müller (1906a) in his original description of the species reported the lengths of females and males from the southern Indian Ocean to be even bigger at 1.9 mm and 1.85 mm, respectively. So are these Indian Ocean specimens conspecific with the Atlantic specimens? This question cannot be resolved until specimens from the Indian Ocean can be examined. In addition, Chavtur (1976) reported a relatively small male (1.4 mm) from the N.E.Pacific.
There are similar problems with M. macromma . This species is either mesopelagic or bathypelagic. Müller’s (1906a) original description is inadequate by modern standards, and several of the subsequent descriptions are little better. Müller (1906a) reported it both from between 24°N and 2°S in the Atlantic and from 13°N and 2°S in the Indian Ocean but failed to specify a type locality. Deevey’s (1974) Hudson 70 material of Conchoecia macromma may have been collected from similar water masses as Müller’s (1906a) Atlantic material, but from much further south (40°S). She reported lengths were longer and she also illustrated (p. 364, fig. 5) HC2 to be 13–14% CH (visà-vis 25% in Müller’s (1906a) illustration), and LC2 to be 35%CL (vis-à-vis 24% in Müller’s (1906a) illustration). Deevey also illustrates the maximum carapace height as being posterior to midlength (vis-à-vis at midlength in Müller’s (1906a) illustration). These differences may well be significant so we are designating her 1974 material as M. sp 1. aff. C. macromma .
Deevey (1982a) reported on a single female specimen from the Southern Ocean , which also deviates from Müller’s type description as follows :
1. The carapace is longer 1.34 mm (vis-à-vis 1.00– 1.07 mm) and higher (55–60% of length).
2. HC2 is 21–22% of the height down the posterior margin (vis-à-vis 25%).
3. The length of e-seta of the A1 is double or more the lengths of the limb and a- to d-setae (vis-à-vis 1.5).
This Southern Ocean female also differed from M. sp 1. aff. C. macromma in that LC2 is shorter (20%CL visà-vis 35%CL). Hence we designate Deevey’s Southern Ocean specimen as M. sp 2. aff. C. macromma .
Poulsen (1973) described and illustrated a single female of Metaconchoecia macromma collected at Dana station 3613 in the S.W. Pacific at 22° 43’S, 166° 06’E. His description also deviates from both Müller’s type description and both of Deevey’s descriptions. Poulsen’s specimen differs from Müller’s type description as follows GoogleMaps :
1. The carapace is longer (1.2 mm) and higher (>50%CL).
2. The rostrum is relatively large (LC3 is 12%CL vis-à-vis 6%).
3. The maximum carapace height is posterior to midlength.
4. HC2 is 24% CH.
5. LC2 is 30%CL.
6. The A1 e-seta is twice the length of the limb and 2.5–3x times the lengths of the a- to d-setae.
7. The structure of the capitulum is different.
We designate Poulsen’s specimen as M. sp 3. aff. C. macromma , noting that it is most similar to M. sp 1. aff. C. macromma , except for the rostrum is larger, and LC2 is shorter. It differs from M. sp 2. aff. C. macromma in the positions of the openings of the asymmetric glands (especially the LAG), its rostrum being larger (6% in M. aff. macromma 2) and the A1 e-seta being relatively longer (2.5–3x the lengths of the a- to d-setae).
Finally, Conchoecia macromma of Angel (1981b) shows only minor differences to the specimens described by Müller (1906a). His material was collected at 0° 23°W (although this is not stated in the publication), which is within the latitudinal range of Müller’s Atlantic material. Since Müller (1906a) neither designated a type locality, nor cited where the specimens he illustrated were collected, it is unclear how significant the differences are between his description and Angel’s. So were Müller’s drawings based on specimens from the Indian Ocean? It is impossible to tell until further material is examined from the Indian Ocean. So here we designate Angel’s (1981b) material as M. sp 4. aff. C. macromma . There are differences in the morphology of the A1, the c-seta of Angel’s male is shorter (three-quarters the combined lengths of segments of the third, fourth and fifth segments vis-à-vis subequal in M. macromma s.s.), the e-seta armature consists of 14 pairs of somewhat short spines (vis-à-vis 15 pairs of longer spines in M. macromma s.s.) and the seta is about double the length of the limb (vis-à-vis 1.5x in M. macromma s.s). Subsequent measurements of Angel’s material show the female e-seta is 1.7x the length of the limb and 2.2x as long as setae a–d (vis-à-vis 1.5x and 1.5x respectively in M. macromma s.s.). His unpublished measurements of 25 specimens are slightly less extreme, giving the mean lengths of the e-seta, limb and a- to d-setae as 27%, 16% and 13%CL, respectively. The carapace lengths of Angel’s material are consistent with Müller’s (females 0.98–1.16, n = 36; males 0.96–1.10, n = 37), but are smaller than reported for the other forms. Table 3 summarizes the variations in the carapace characteristics of all these forms. Figure 5 View FIGURE 5 reproduces the original published drawings of the female carapaces at identical scales and clearly demonstrates that neither Deevey’s nor Poulsen’s specimens are likely to be the same as Müller’s. Clearly a further re-appraisal is needed.
Distribution. Atlantic Ocean: found between 42ºN and 44ºS ( Müller 1906a, 1908; Iles 1953; Deevey 1968, 1974; Angel 1979, 1981b, 1983a). Indian Ocean: known from between 13ºN and 29°S ( Müller 1906a; Leveau 1967; Gooday 1981). Pacific Ocean: occurs in the area of the Kurile-Kamtchatka and Aleutian Trenches ( Rudjakov 1962; Chavtur 1977a, b, c, 1992), China Seas ( Chen et al. 1983; Chen & Lin 1995) and from 22ºS to 40ºS ( Poulsen 1973; Martens 1979; Deevey 1983). Southern Ocean: recorded as far south as 60–64ºS in the Pacific sector ( Deevey 1982a, 1983). The described overall depth range is 200–8000m, but once again the maximum depth cited may be misleading as many of the records are from samples collected by open vertically hauled nets. (Note: neither Deevey (1982a) nor Poulsen (1973) had any males, so males of M. spp 2 and 3. aff. C. macromma are unknown).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |