Diprotodon, Owen, 1838
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2008.00387.x |
|
persistent identifier |
https://treatment.plazi.org/id/03DC87E5-D159-FFAF-223D-FF15FDDAF923 |
|
treatment provided by |
Felipe |
|
scientific name |
Diprotodon |
| status |
|
TAXONOMY OF DIPROTODON
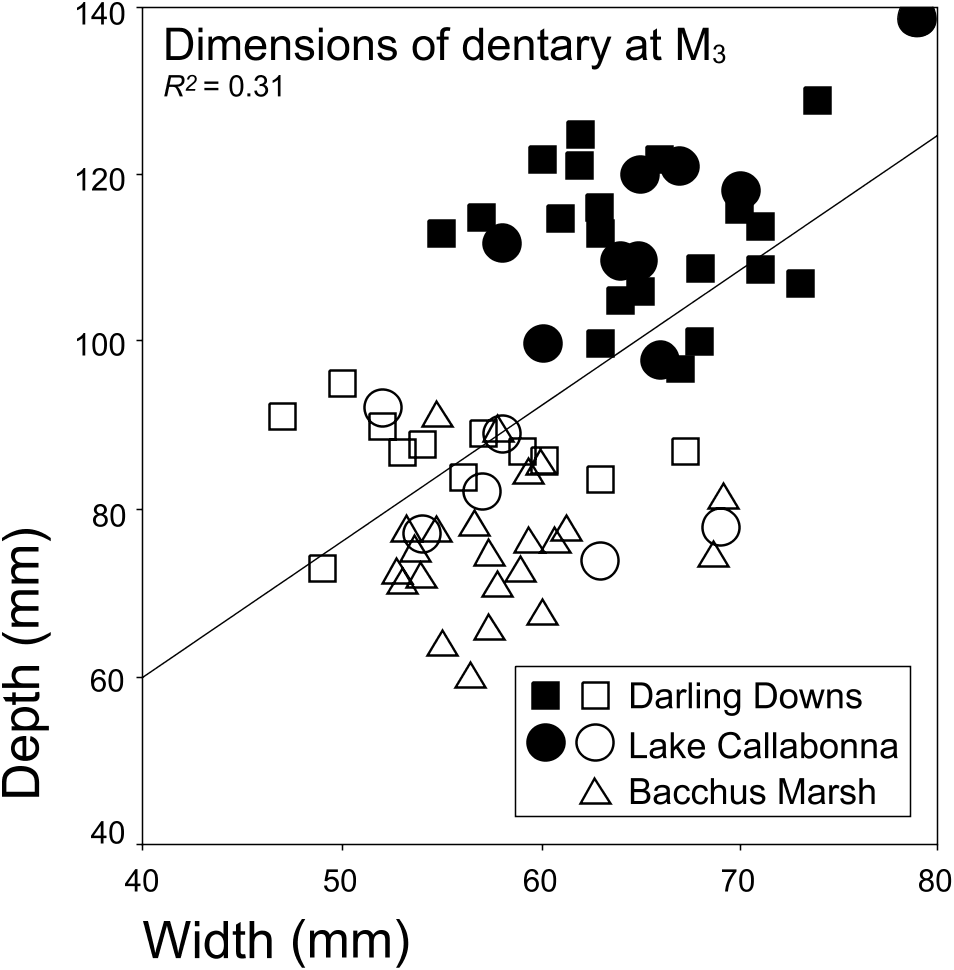
Differences in dentary size and morphology exist within several of the assemblages examined ( Fig. 8 View Figure 8 ). However, those morphologies are also exhibited in all other assemblages where dentaries are well preserved. Where dentaries can be unequivocally assigned as either large- or small-form Diprotodon , associated cheek teeth reflect a bimodal size distribution, although a considerable degree of overlap exists between measurements (e.g. Table 1, Figs 10 View Figure 10 , 11 View Figure 11 , 14 View Figure 14 , 15 View Figure 15 ). On the basis of dental morphological and morphometric data, there is currently little basis to discriminate more than one Diprotodon morphospecies within or between the Darling Downs, Reddestone Creek, Myall Creek, Lake Callabonna, Lancefield Swamp and Bacchus Marsh assemblages. Thus, dimorphism in Diprotodon body size, as suggested by dentary and teeth size, can be explained as being purely size-related, not reflecting an ontogenetic series. Therefore, following criteria established in the Introduction, the differences between the large- and small-form Diprotodon , within and between all major assemblages examined, reflect intraspecific differences, i.e. sexual dimorphism within a single morphospecies, rather than interspecific differences. That interpretation has significant implications for the taxonomy of most currently recognized Diprotodon species. All studied material is considered here to belong to Diprotodon optatum Owen, 1838 (see Appendix 1). Diprotodon australis Owen, 1844 , D. annextans McCoy, 1861 , D. minor Huxley, 1862 , D. longiceps McCoy, 1865 , D. loderi Krefft, 1873a , D. bennettii Krefft, 1873b (nec D. ‘ bennettii ’ Owen, 1877) and D. ‘ bennettii ’ Owen, 1877 (nec D. bennettii Krefft, 1873b ) are here considered junior synonyms of D. optatum . Generally, most diagnostic features originally suggested for the synonymized species were related to size or slight morphological variations between single, isolated specimens collected from isolated geographical areas. The range of dental morphological and morphometric variation exhibited in both large- and small-form individuals, and for previously erected Diprotodon species , is, however, easily encompassed within single Diprotodon assemblages of the eastern Darling Downs, Reddestone Creek, Myall Creek, Lake Callabonna, Lancefield Swamp and Bacchus Marsh. A major implication of this taxonomic interpretation is that only one species of Diprotodon , rather than eight, lived during, and suffered extinction, sometime in the late Pleistocene.
Sexual dimorphism is characteristic of a wide variety of extant mammals and is particularly prevalent in megaherbivores. For example, within extant African and Asian elephants, adult males may grow to be almost twice as heavy as adult females (Owen- Smith, 1988). While there are obvious differences in the shape of the skull, dentary and tusk between genders ( Haynes, 1991), cheek teeth are morphologically identical ( Roth, 1992). Although male elephants have slightly larger cheek teeth than females, the difference is not marked ( Roth, 1992). Within large extant sexually dimorphic marsupials such as the grey kangaroo, there is also a similar overlap in teeth dimensions between sexes ( Fig. 13 View Figure 13 ), but with no major differences in dental morphology. An identical relationship is obvious between the large- and small-form Diprotodon ( Figs 4 View Figure 4 , 7 View Figure 7 ; Table 1), thereby providing additional support for the interpretation of sexual dimorphism.
SPATIAL AND TEMPORAL DISTRIBUTION
Stirling & Zietz (1899), Gill (1954), Murray (1991) and Molnar & Kurz (1997) suggested that the smallform Diprotodon was less common, and geographically restricted in comparison with the large-form Diprotodon . However, the proportions of large- to small-form individuals in the Darling Downs, Lake Callabonna and Lancefield Swamp Diprotodon assemblages are fairly close to parity or slightly biased to the small form, suggesting that both size classes were sympatric, equally abundant and widespread. For reasons suggested above, the difficulties involved in accurately identifying a large- or smallmorph D. optatum may be polarized when analysing only fragmentary and isolated remains, and have previously led to misinterpretations regarding the distribution of Diprotodon size classes.
As here recognized, Diprotodon optatum was extremely wide-ranging, with a near continent-wide distribution ( Fig. 2 View Figure 2 ). Thus, the Pleistocene geographical distribution of D. optatum was similar to that for extant megaherbivores such as African elephants. In the historical period, African elephants had a nearcontinental distribution and occupied almost every habitat south of the Sahara (although, more recently, the geographical distribution has decreased significantly) ( Douglas-Hamilton & Michaelmore, 1996). Like African elephants, D. optatum was probably also a habitat generalist. That interpretation is supported by stable isotope data that suggest that Diprotodon had the ability to utilize a variety of C3 and C4 food resources over wide geographical areas ( Gröcke, 1997).
Although only one-half of the sites examined in this paper are well dated, there is little evidence of significant temporal morphological and morphometric variation between Diprotodon optatum assemblages. Given the trend for diprotodontoids to increase in body size over time from the early Miocene to the late Pleistocene ( Murray, 1991), the similarities in teeth size between all Diprotodon assemblages examined suggests that those localities may be temporally coeval. Drawing from analytical dating of Darling Downs, Lake Callabonna and Lancefield Swamp deposits ( Gill, 1978; Gillespie et al., 1978; Van Huet et al., 1998; Roberts et al., 2001; Price, 2005; Price & Sobbe, 2005; Price et al., 2005; Webb et al., 2007), that interpretation would suggest that the Bacchus Marsh, Myall Creek and Reddestone Creek assemblages may also be late Pleistocene. However, that hypothesis may obscure potential physiological geographical body size effects that could relate to spatial habitat or climatic differences, such as Bergmann’s rule ( Bergmann, 1847; Meiri & Dayan, 2003). Bergmann’s rule suggests that animals in cooler climates tend to be larger than congeners that occur in warmer climates ( Bergmann, 1847). Such geographical morphoclines may be the result of a taxon’s response to latitudinal differences in primary productivity and seasonality, or physiological adaptations to ambient temperature, although the mechanisms driving the underlying patterns remain unclear ( Meiri & Dayan, 2003; Blackburn & Hawkins, 2004). Regardless of the cause, for extant Australian marsupials, Bergmann’s rule suggests that species in southern (cooler) regions are larger than species that occur in northern (warmer) regions. Yom-Tov & Nix (1986) observed Bergmann-type responses of taxa within several Australian marsupial groups. For D. optatum , there does not appear to be a latitudinal morphocline in body size ( Figs 14 View Figure 14 , 15 View Figure 15 ). However, that hypothesis relies on the assumption that all D. optatum assemblages examined in this study are temporally coeval. Ideally, that hypothesis can be tested only by securely dating material from all Diprotodon localities examined.
Diprotodon optatum is so far reliably known only from Pleistocene deposits. However, Tedford (1994) reported Diprotodon from Pliocene deposits of Fisherman’s Cliff & Dog Rocks, Victoria. Archer (1977) regarded those records as doubtful, and Mackness & Godthelp (2001) suggested that such specimens may actually belong to Euryzygoma . The youngest reliable records place Diprotodon in late Pleistocene deposits ( Roberts et al., 2001; Pledge, Prescott & Hutton, 2002).
PALAEOBIOLOGY
Mating strategy
If it is provisionally accepted that the large-form Diprotodon optatum is male and small form is female, there are some very interesting implications for behaviour and life strategy. In many large extant marsupials such as kangaroos, there is a strong correlation between body size and degree of sexual dimorphism. That is, larger-sized species tend to be more sexually dimorphic than smaller-sized species ( Jarman, 1989). That is also evident here, where the degree of dimorphism within the ~2500-kg D. optatum (~4–17% difference in cheek teeth morphometrics between genders) is greater relative to that of the extant ~50-kg grey kangaroo (~4–10% difference in cheek teeth morphometrics between genders). For ungulates, Loison et al. (1999) suggested that allometry was unimportant in shaping sexual dimorphism, but rather, the degree of polygyny can almost entirely account for the relationship between increasing size dimorphism and body size. Most notably, polygynous mating systems are dominant in all sexually dimorphic extant megaherbivores ( Owen-Smith, 1988). Thus, by drawing analogy to extant groups, it is most likely that D. optatum exhibited a polygynous breeding strategy.
Social groups
In cases of large-sized extant taxa exhibiting extreme sexual dimorphism, mature males and females commonly live in separate social groups ( Owen-Smith, 1988). Importantly, the frequency of sexual segregation increases with increasing levels of sexual body size dimorphism ( Mysterud, 2000). Such gender segregation may be a consequence of differential exploitation of resources (i.e. ecological segregation) relating to asynchrony of activity budgets between sexes ( Isacc, 2005). However, reasons for ecological segregation are less clear. There is a clear link between body size and the nutritional ecology of large herbivores ( Demment & Van Soest, 1985). Largersized herbivores can survive on lower quality food than smaller-sized herbivores. Thus, within largesized taxa exhibiting extreme sexual dimorphism, larger-sized males may accept lower quality forage than smaller-sized females ( Demment & Van Soest, 1985; Mysterud, 2000). Alternatively, gender segregation may affect both performance and survival of the sexes. Large males may select high-quality forage in order to improve body condition and growth, factors that may affect fighting ability and therefore reproductive success ( Mysterud, 2000). Females may select habitats that maximize their ability to raise young ( Mysterud, 2000). Haynes (1991) observed that in modern bone assemblages of extant sexually dimorphic megaherbivores such as elephants, adult females and young are always the most abundant, and males are comparatively rare. For sexually dimorphic ungulates, Ruckstuhl & Neuhaus (2002) suggested that gender segregation is related to incompatibilities of activity budgets and movement rates. Males and females are commonly found in the same geographical area (thus do not segregate by habitat), select the same plants, but have differential temporal patterning of foraging ( Ruckstuhl & Neuhaus, 2002). Examining food resource partitioning between the large- and small-form D. optatum may be possible using stable isotope analysis. However, current research in such dietary studies has not differentiated between large- and small-form individuals (e.g. Gröcke, 1997).
By drawing analogy to large-sized extant sexually dimorphic herbivores, Diprotodon optatum probably did exhibit gender segregation among social groups. However, extracting data relating to social structure from the examined Diprotodon assemblages is difficult due to the allocthonous nature of most deposits. The Bacchus Marsh deposit is one of the most autochthonous deposits examined in this study, and the Diprotodon assemblage indicates that the small form (?female) was abundantly represented (N = 18 individuals) whilst the large form (?male) was absent. Other assemblages with large numbers of individuals such as the Darling Downs (N> 50 individuals) are also biased (~ 1.4:1) towards small-form (?female) individuals. Thus, such evidence provides some support for gender social segregation within D. optatum herds.
EVOLUTION
Diprotodon optatum and the Pliocene Euryzygoma dunense ( de Vis, 1888a) are united within diprotodontines by the unique morphology of the basicranium where posterior elongation and fusion of the postglenoid process with the tympanic process and mastoid–squamosal forms a complete auditory meatus ( Murray et al., 2000). However, D. optatum is more derived than E. dunense by possessing: (1) higher-crowned molars; (2) molar lophs that are angled more perpendicular relative to the length of the dentary; and (3) by being larger (an example of phyletic giantism; sensu Gould & MacFadden, 2004). Additionally, although highly variable, D. optatum P 3 morphology where the paracone and metacone are divided is an autapomorphy within the Diprotodontinae ( Archer, 1977) . The morphological and morphometric variability of D. optatum premolars may be the result of their small relative size ( Tables 1, 2), associated with their presumed slight functional importance to the animal ( Marcus, 1976). The interpretation here of sexual dimorphism within Diprotodon raises the possibility that sexual dimorphism also may have occurred in other members of the family. Thus far, sexual dimorphism has previously only been reported in the zygomaturine Neohelos stirtoni ( Murray et al., 2000) .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Diprotodon
| Price, Gilbert J. 2008 |
Diprotodon optatum
| Owen 1838 |
Diprotodon
| Owen 1838 |
Diprotodon
| Owen 1838 |
Diprotodon optatum
| Owen 1838 |
D. optatum
| Owen 1838 |
D. optatum
| Owen 1838 |
D. optatum
| Owen 1838 |
Diprotodon
| Owen 1838 |