Astyliasula sarawaca ( Westwood, 1889 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4291.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:28772C8C-1E20-4A92-A9BD-1F5D016BD981 |
|
DOI |
https://doi.org/10.5281/zenodo.6050960 |
|
persistent identifier |
https://treatment.plazi.org/id/03DC8124-1806-A162-FF6B-FD94FCC166A6 |
|
treatment provided by |
Plazi |
|
scientific name |
Astyliasula sarawaca ( Westwood, 1889 ) |
| status |
stat. nov. |
Astyliasula sarawaca ( Westwood, 1889) View in CoL stat. rev. & n. comb.
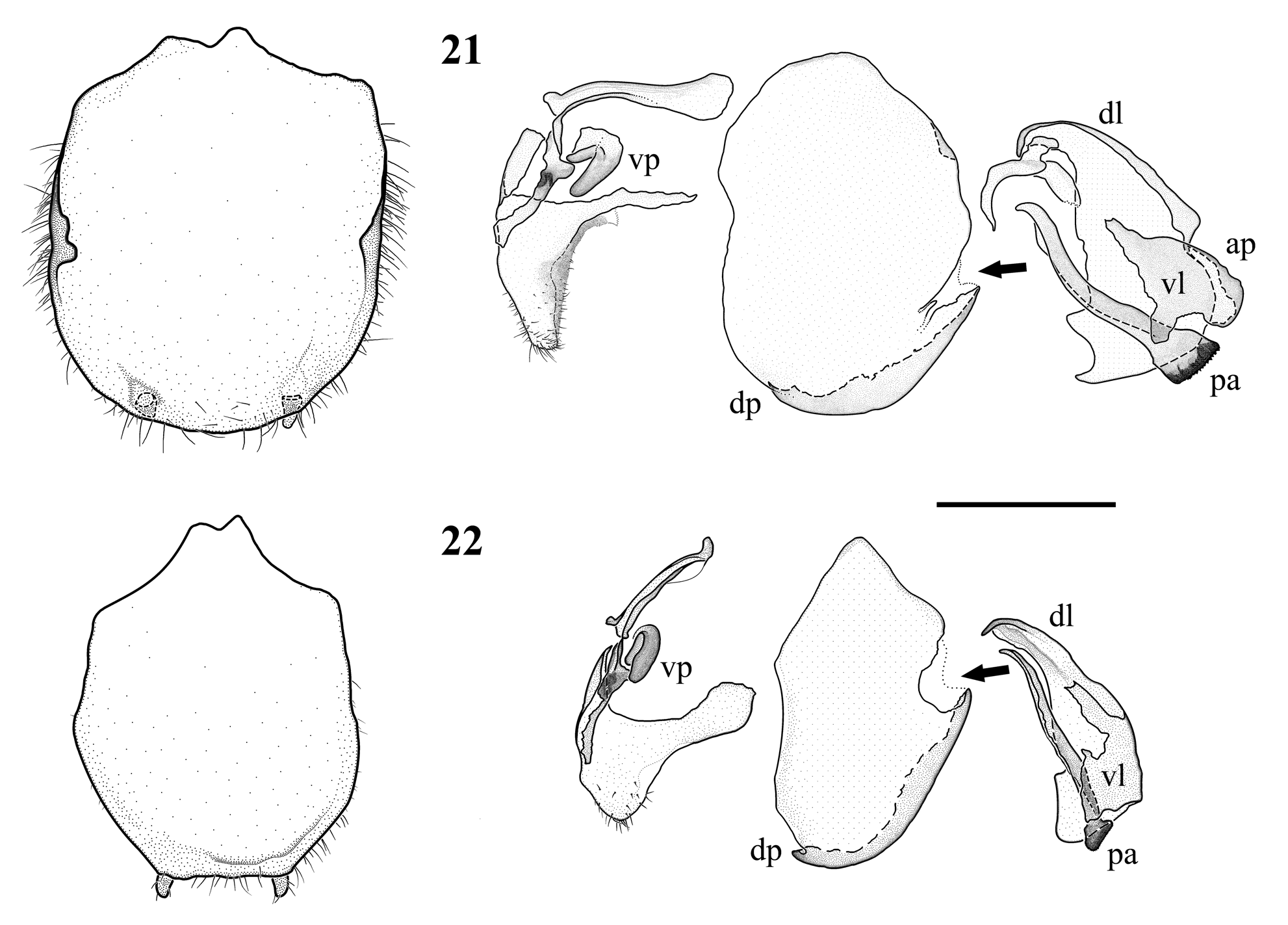
( Figs 5–6 View FIGURES 1 – 8 , 12, 16 View FIGURES 9 – 20 , 23–36 View FIGURES 23 – 25 View FIGURES 26 – 32 View FIGURES 33 – 36 , 53 View FIGURES 53 – 57 , 62 View FIGURES 60 – 62 )
Hestias sarawaca Westwood 1889: 44 , pl. 11, fig. 1 (♀)
Pachymantis sarawaca ( Westwood, 1889) . In: Kirby 1904: 288 (partim).
Type material. Holotype: ♀, Sarawak, A. R. Wallace leg., coll. Saunders (OUMNH-466; pinned) ( Figs 33–36 View FIGURES 33 – 36 ).
Additional material. 1 ♂, Indonesia, NE Sumatra, Dolok, Merangir , 180 m, 04.–14.III. 1967, E. W. Diehl leg. (SMNK-03643; pinned) ; 1 ♂, Malaysia, Borneo, Sarawak, Gunung Santubong, Hotel “ Damai Lagoon ”, N 1.47° –E 110.19°, lux, 15.X.2000, H. Karbaum leg. (SMNK-03630; pinned); 1 ♂, Brunei, Borneo, Ulu Temburong National Park, Kuala Belalong Field Studies Centre , II.2013, O. Konopik leg. (genitalia preparation Schwarz No. 59) (CS; pinned) ; 1 ♂, same place (genitalia preparation Schwarz No. 51) (SMNK, ex. CS; pinned); 1 ♂, Malaysia, Borneo, Sabah, Melalap , 26.–30.I.2004, M. D. Mahadimenakbar leg. ( BOR; pinned) ; 1 ♂, Malaysia, Borneo, Sabah, Keningau distr., Trus Madi Forest Reserve , N 05°26’35” –E 116°27’05”, 1250 m, mixed dipterocarp forest, 25.– 26.V.2014, E. Shcherbakov leg. (ES; pinned); 3 ♂♂, Malaysia, Borneo, Sabah, Keningau distr., Trus Madi Forest Reserve , 1160 m, 19.–20.III.2012, A. Klimenko leg. ; 1 ♀, Pachymantis sarawaka (sic!) Westw . ♀ = phyllopus De Haan (handwritten), Borneo-Exped. Dr. Nieuwenhuis 1894, Mahakkam riv. (RMNH-INS 968168; pinned).
Redescription. Male. Head large, triangular , distinctly depressed between compound eyes. Compound eyes roughly pentagonal, exophthalmic. Vertex nearly flat, with a pair of shallow depressions, separated from the ocellar area by a sulcus connecting the sockets of compound eyes, and from the sockets of compound eyes by parietal sulci. Ocelli elevated on sockets, forming an isosceles triangle. Frontal ocellus nearly round, lateral ocelli oval, slightly larger than the frontal ocellus. Scutellum transverse, its ventral margin slightly concave, lateral parts of dorsal margin concave, converging into a rounded and slightly protruding apex. Surface of scutellum with two paramedian tubercles and two lateral carinae. Clypeus elevated medially, with a very thin carina ventrad.
Pronotum rhomboidal in dorsal perspective, anterior margin widely rounded, posterior margin truncate. Lateral margins of prozona very indistinctly serrulated, of metazona just posteriad supracoxal furrow with a small triangular spine on each side. Prozona and metazona dorsally smooth, laterally covered by long setae. Prozona with a shallow sulcus following the curvature of the anterior margin, with a pair of sinuous paramedian ridges, and a very low median ridge in its posterior half. Metazona about the same length as prozona, separated from the latter by deep transverse supracoxal furrow, with a low median ridge and a pair of flat, rounded tubercles at its posterior margin. Lateral margins of metazona sharply bent dorsally in lateral perspective. Meso- and metathorax similar in size, covered by long setae, metathorax ventrally with a cyclopean ear of the DK type.
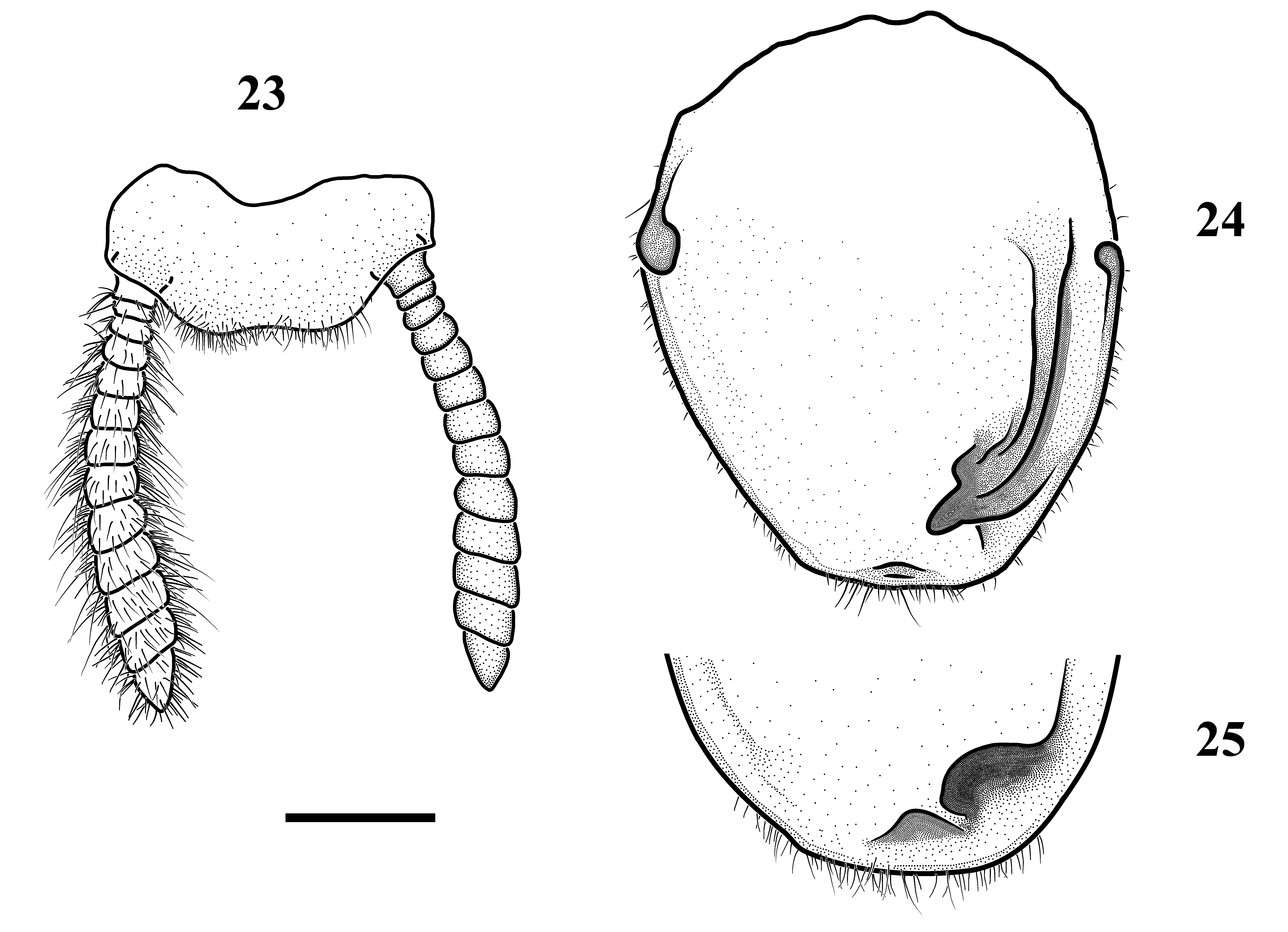
Forelegs. Fore coxa dorsally with 5–7 small oblique spines and several smaller tubercles, ventrally with 15–18 small tubercles roughly arranged in two rows. Apical lobes adjacent. Fore femur ( Fig. 12 View FIGURES 9 – 20 ) wide, with extremely dilated, lamellar, nearly smooth dorsal edge, ratio dilated/ventral part 1.5–1.6. Surface of dilated part irregularly undulated. Posterior surface of fore femur with multiple small tubercles. Ventral border with 12–15 antero-ventral, 4 discoidal, and 4 postero-ventral spines. Genicular lobes with a spine each, postero-ventral spine being much larger than antero-ventral one. All spines very short. Antero-ventral row with alternating longer and shorter spines, the first always short, the last always long, of the formula iIiIiIiIiIiI(ii)I. Discoidal spines arranged in a curved row, the proximal one at some distance from the remaining, aligned with antero-ventral row; size of the spine as follows: 3>2>4>1. Postero-ventral spines emerging from the cuticle flush with surrounding region, almost equal in length, the proximal two closer to each other than the remaining. Ventral margin of the femur between posteroventral spines serrulate, with small pit inside the triangle formed by the two proximal-most postero-ventral spines and distal-most discoidal spine to accommodate the distal-most postero-ventral spine of fore tibia. Fore tibia dorsally slightly convex, ventrally with 13–16 antero-ventral and 15–17 postero-ventral spines. Tibial spines regularly elongating towards apex. Antero-ventral spines spaced apart, postero-ventral spines short and decumbent, except the most distal ones. Last postero-ventral spine particularly long compared to the others.
Mid and hind legs cursorial, pilose, without lobes. Femur with genicular spine at apex. Apex of tibia with a triangular elongation and two apical spines ventrad to elongation.
Wings well developed. Tegmen elongate, moderately wide, with rounded apex. Costal field about 1/3 of tegmen width at basal third of the tegmen’s length, strongly narrowing distally, with irregular (“tree-shaped”) cross-veins. Towards the apex, cross-veins form only one row of cells between main veins. Stigma indistinct. Hind wings oxygonal, with rounded apex.
Abdomen long, with constant width and simple, transverse tergites and sternites. Supra-anal plate ( Fig. 23 View FIGURES 23 – 25 ) trapezoidal, apex very weakly concave at the middle. Cerci strongly setose, with 13–14 recognizable cercomeres, first cercomere consisting of two fused cercomeres. Basal cercomeres rounded in cross-section, becoming wider and more ellipsoid towards apex. Subgenital plate ( Fig. 24–25 View FIGURES 23 – 25 ) strongly setose, anteriorly semicircular, with extremely small anterior arms, posterior margin trapezoidal. Styli completely absent. Dorsal corners slightly asymmetrical, strongly sclerotized. Right half of posterior part dorsally with a long, curved sclerotized ridge, and a lower, less sclerotized ridge mediad the first one. Both ribs posteriorly fused, forming a large, strongly sclerotized dorsal process. A smaller, less sclerotized process is situated to the left of the first one, almost at the apex of the subgenital plate.
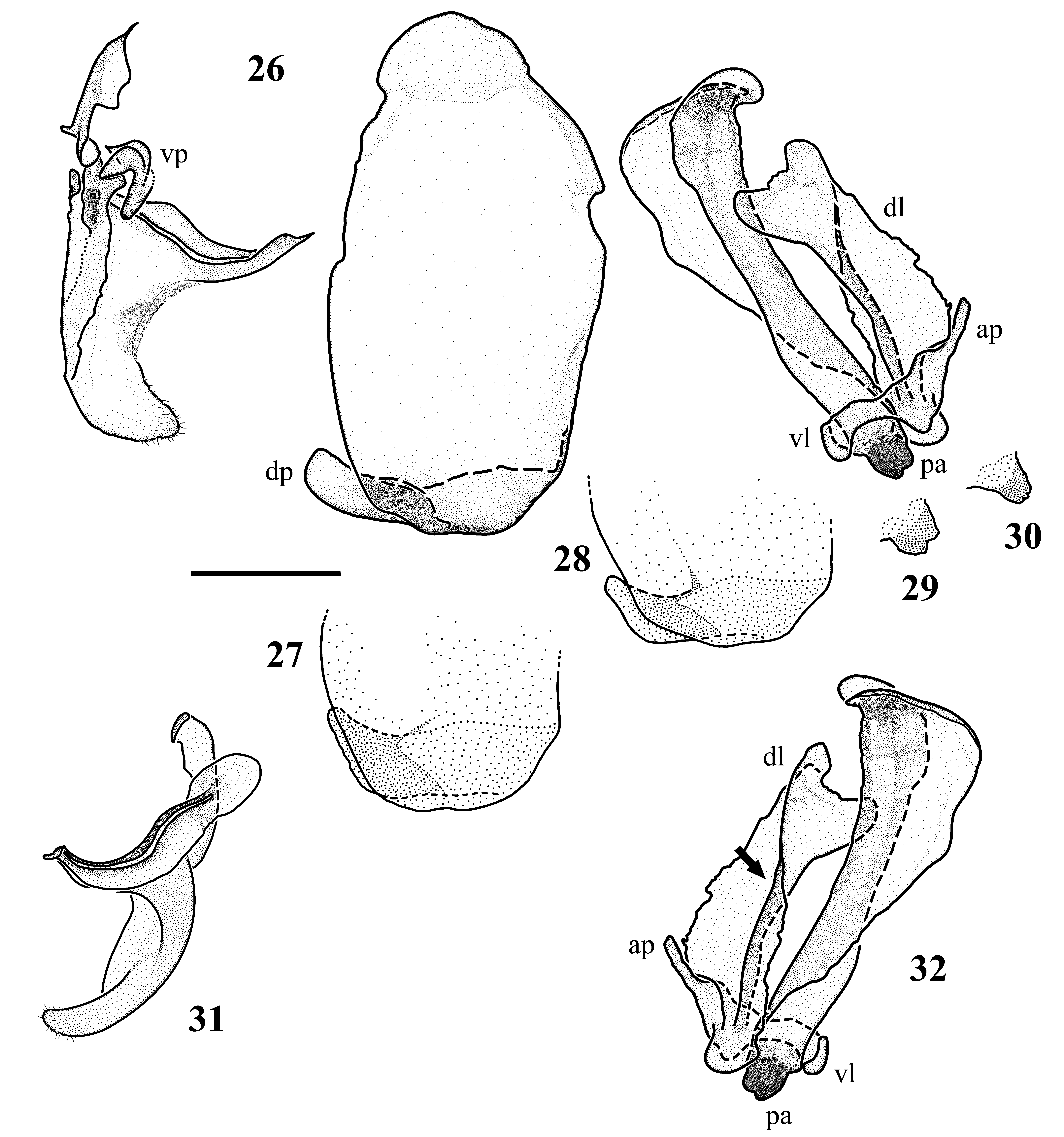
Genitalia ( Figs 26–32 View FIGURES 26 – 32 ). Ventral phallomere roughly oval. Distal process very large, shovel-like, moderately sclerotized, narrowing dorso-ventrally toward the apex. Dorsal lamina of the left phallomere sharply folded to the right along the middle, posteriorly fused with the base of apical process. Apical process weak, thin, round in crosssection. Phalloid apophysis rectangular to trapezoidal, strongly sclerotized, slightly bifurcate at apex. Right phallomere comma-shaped, with pilose apex, its dorsal lamina with anterior edge bent dorsad, the lamellar overgrowth with a very narrow transverse pocket opening anteriorly ( Fig. 31 View FIGURES 26 – 32 ). Ventral process simple, digitiform.
Coloration. Main color brown. Head brownish, eyes dorso-ventrally banded, vertex blackish. Palps darkened ventrally. Antennae brownish, third segment with dark annulation.
Pronotum mottled with dark, lighter along supracoxal furrow. Prozona anteriad paramedian ridges and median area of metazona blackish. Furcasternum and mesosternum shiny black. Anterior side of fore coxae dark brown proximally, shiny black apically. Trochanter shiny black. Posterior side of fore femora yellowish-brown, tubercles dark. Anterior side of fore femora shiny black at base, black coloration extending as a broad band along the dorsal margin, from the dorsal half of the claw groove until the distal third of the femur. Ventral margin of the fore femur with two black parallelogram-shaped spots, the first at middle of femur from sixth to eighth antero-ventral spine, the second at the base of the 2–3 most distal antero-ventral spines; both markings extending to half the width of the non-dilated part of the femur. Remaining parts of fore femora yellow to orange. Discoidal spines reddish-brown, antero-ventral spines dark. Fore tibiae yellowish-brown posteriorly, black anteriorly. Fore tarsus black ventrally.
Meso- and meta-femora and tibiae mottled with black, proximal third of anterior side of meso femora with a black stripe.
Wings hyaline, with dark apex. Costal field of tegmen subhyaline, spotted with dark. Discoidal field with two irregular smoky markings, a more distinct one at middle, and a smaller subapical one. Veins partially dark. Alae hyaline with spotted anterior margin, a subhyaline area near posterior angle, and dark apex.
Females. Like A. phyllopus , but slightly smaller and with comparatively shorter pronotum and shorter mid and hind legs ( Figs 6 View FIGURES 1 – 8 , 33–36 View FIGURES 33 – 36 ), while head and fore leg size fall into the range of the former. Anterior side of fore coxae brownish, becoming slightly darker towards apex. Pattern of fore femora similar to males ( Figs 16 View FIGURES 9 – 20 , 36 View FIGURES 33 – 36 ), but blackish pattern along dorsal margin less pronounced in the Leiden specimen. Wings about as long as abdomen, reaching slightly beyond supra-anal plate. Tegmina subopaque, like in phyllopus with two subhyaline patches at the posterior margin of the distal-most part of the discoidal field. Costal field slightly less than 1/3 of tegmen width, reaching greatest width just proximad of middle of tegmen, contrary to phyllopus where the costal field attains 1/3 of tegmen width at about its proximal third, then gradually tapers towards subcostal vein. Hind wings except the apex strongly infumate with light veins. Abdomen widened, fusiform.
Measurements, in mm. Male: body length 22.0–23.0; head width 4.2–4.5; head length 3.5–3.7; antennae length 15.0–16.3; pronotum length 3.5–4.1; pronotum width 3.0–3.5; prozona 1.5–2.0; metazona 1.9–2.1; fore coxa length 5.0–6.5; fore femur length 7.0–8.1; fore femur width 4.8–5.3; fore tibia length 4.0–6.1; meso femur length 4.0–4.8; meso tibia length 3.5–4.5; hind femur length 5.0–5.4; hind tibia length 5.0–6.0; tegmen length 20.0–23.0; tegmen width 5.6–6.2.
Female (RMNH specimen): body length 29.4; head width 5.4; head length 5.0; pronotum length 5.6; pronotum width 4.7; prozona 2.8; metazona 2.8; fore coxa length 8.5; fore femur length 10.6; fore femur width 6.8; meso femur length 4.8; meso tibia length 5.1; hind femur length 6.1–6.2; hind tibia length 7.1; tegmen length 21.3; tegmen width 8.1.
Variability. In this species the ratio pronotum length/width of males usually lies between 1.16–1.18, but here also there is an aberrant specimen from Sarawak that has a particularly slender pronotum which is 1.23 times longer than wide. The ratio in females is 1.11–1.19.
Diagnosis. A. sarawaca is closely related to A. phyllopus , which is evidenced by very similar fore-femoral patterns and male genitalia. It differs from A. phyllopus by smaller size, a shorter pronotum, a slightly shorter apical process, comparatively shorter mid and hind legs in both sexes, and the pattern of the wings in males.
Because of occasional outliers, the ratio pronotum length/width (e. g. Agabiti et al. 2010) can be only used as a rule of thumb to differentiate between males of the two species: in A. sarawaca this ratio is usually below 1.2, while it is at least 1.2 in A. phyllopus . Female patterns are more clearly defined, sarawaca having a ratio below 1.2, while it is 1.29 to 1.33 in phyllopus .
On the other hand, males of the two species can be clearly distinguished by the color pattern of the wings, which is constant and not influenced by the intensity of the body coloration nor by body size and proportions. In A. phyllopus both pair of wings are heavily mottled with dark ( Figs 3 View FIGURES 1 – 8 , 57 View FIGURES 53 – 57 ), whereas in A. sarawaca this pattern is strongly reduced, with only the dark apex, the two transverse maculations on the tegmen, and the smoky posterior corner of the hind wings remaining ( Figs 5 View FIGURES 1 – 8 , 53 View FIGURES 53 – 57 ). Additionally, A. sarawaca males differ from A. phyllopus males by a slightly shorter apical process, significantly smaller body length (22.0–23.0 mm in A. sarawaca males versus 26.5–29.2 mm in A. phyllopus males), and shorter tegmina (20.0–23.0 mm in A. sarawaca males versus 26.5–28.9 mm in A. phyllopus males). As for the females, in A. sarawaca the tegmina are 3.4–3.8 times as long as the pronotum, while in A. phyllopus this value attains only 3.1–3.2. Their costal field is differently shaped than that of A. phyllopus females (see above).
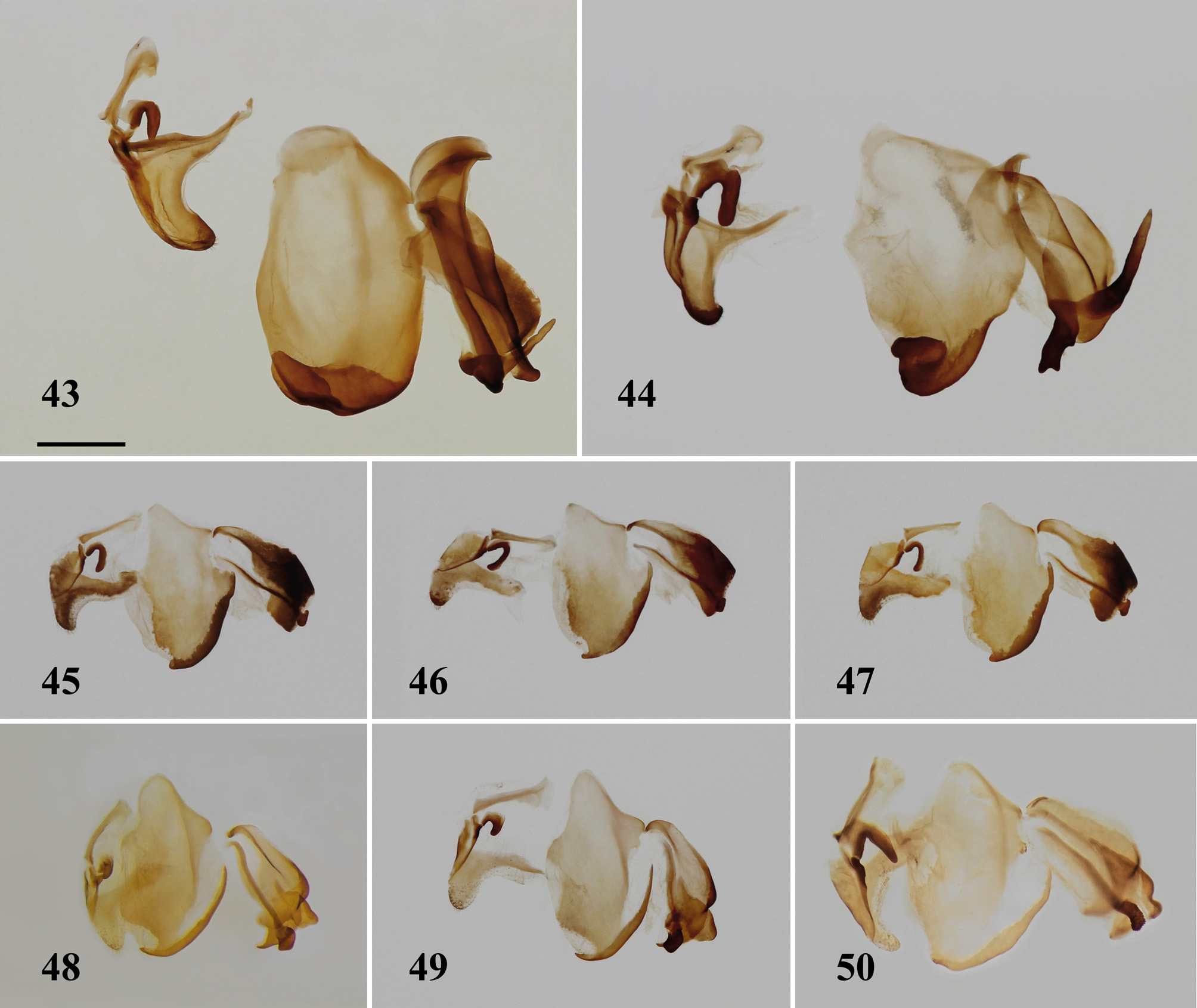
Males of A. sarawaca resemble those of A. javana in having black fore coxae and largely hyaline wings with a black apical spot. Both sexes of A. sarawaca differ from A. javana in the foreleg pattern, the latter having but one black maculation at the antero-ventral margin. A. sarawaca may be distinguished from continental species of Astyliasula by its relatively short and narrow apical process, and a more elongate distal process with smooth, gently curved posterior edge (compare Figs 26–28 View FIGURES 26 – 32 with Fig. 44 View FIGURES 43 – 50 ).
Remarks. This species was described after a female from Sarawak ( Figs 33–36 View FIGURES 33 – 36 ) deposited in the OUMNH. All females from Borneo resembling the type figured by Westwood (1889, p. 22 & 44, pl. 11, fig. 1) have subsequently been assigned to this taxon: Brunner de Wattenwyl 1893: 73 (as Hestias sarawaca ); Shelford 1903: 296, 302, fig. 2; Chopard 1938: 366, 419–420 (as Hestiasula sarawaka ); Kirby, 1904: 288; Werner 1916: 280–281 (as Pachymantis sarawaca ). It was Giglio-Tos (1917, p. 158) who first established the synonymy between Hestias sarawaca and Mantis phyllopus and later (1927, p. 544) assigned the species to Saussure’s genus Hestiasula in concordance with Shelford (1903). This arrangement has been largely followed since (e. g. Ehrmann 2002, Otte & Spearman 2005, Schwarz & Konopik 2014).
However, the type of H. sarawaca and an additional female in the RMNH collection originally identified as H. phyllopus ( Figs 6 View FIGURES 1 – 8 , 16 View FIGURES 9 – 20 ) exhibit different proportions than typical phyllopus females. The pronotal ratio of sarawaca females corresponds to values seen in the males described above, as is the case with the pattern on the fore femora and the proportionally shorter walking legs. Even though the first two characters are also exhibited by occasional phyllopus males, the latter are well distinguishable from the males assigned here to sarawaca by their different tegminal pattern and the other characters listed in the diagnosis.
The brownish fore coxae of sarawaca females contrast with the blackish color of males, and also with the situation in related species, where the color of the coxae does not differ between the sexes. However, the coloration of the fore coxae is not a reliable character speaking against conspecificity of the sarawaca holotype with the males described above, since there are also dark specimens of A. phyllopus (see above, Fig. 9 View FIGURES 9 – 20 ). In such individuals, the usually reddish coxae may be darkened to an extent almost indistinguishable from that of sarawaca . On the other hand, the base of the coxae in the Santubong sarawaca specimen is lighter than the apex and of a dark reddishbrown.
Interestingly, the genitalia of phyllopus and sarawaca are almost identical, which contrasts with the clearly divergent external morphology of the males. This does nor argue against two species being valid, since hymenopodid genitalia are remarkably similar in closely related species, contrary to the inter-generic differences elaborated upon above. For example, the genitalia of Ephestiasula amoena ( Fig. 48 View FIGURES 43 – 50 ) are hardly distinguishable from those of E. pictipes ( Fig. 49 View FIGURES 43 – 50 ), or from those of Hestiasula brunneriana ( Fig. 21 View FIGURES 21 – 22 ) for that matter. Likewise, the genitalia of all Catestiasula specimens studied so far (e. g. Figs 22 View FIGURES 21 – 22 , 45–47 View FIGURES 43 – 50 ) have not yet provided truly unique species-specific characters, unlike the pattern of the foreleg ( Figs 17–20 View FIGURES 9 – 20 ). A lack of reliable species-specific genital characters contrasting with well-differenciated external morphology is also observed in other hymenopodid genera like Sibylla Stål, 1856 ( Roy 1996) , Majangella Giglio-Tos, 1915 ( Svenson & Vollmer 2014) , Anaxarcha Stål, 1877 , Acromantis Saussure, 1870 , and Creobroter Audinet-Serville, 1838 (pers. obs).
Finally, sarawaca males are not too rare in Bornean material, and largely sympatric with phyllopus throughout most of northern Borneo and in parts of Sumatra. Their coexistence thus points to the existence of two welldifferenciated species. Currently, we are not aware of Bornean Astyliasula males which do not fit the diagnoses of the two species described above.
On Trus Madi all specimens of A. sarawaca were encountered near the light traps only during 30 minutes before sunrise, unlike A. phyllopus , which fly much earlier.
| BOR |
Guermonprez Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Oxypilinae |
|
Genus |
Astyliasula sarawaca ( Westwood, 1889 )
| Schwarz, Christian J. & Shcherbakov, Evgeny 2017 |
Pachymantis sarawaca (
| Kirby 1904: 288 |
Hestias sarawaca
| Westwood 1889: 44 |