Polyergus rufescens
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3722.4.5 |
|
publication LSID |
lsid:zoobank.org:pub:C1F59CA8-0F0E-471B-9B2D-26980A002511 |
|
DOI |
https://doi.org/10.5281/zenodo.6150047 |
|
persistent identifier |
https://treatment.plazi.org/id/03DBDC46-FFB1-FF90-4BBE-FD2DFB886C91 |
|
treatment provided by |
Plazi |
|
scientific name |
Polyergus rufescens |
| status |
|
Polyergus rufescens View in CoL
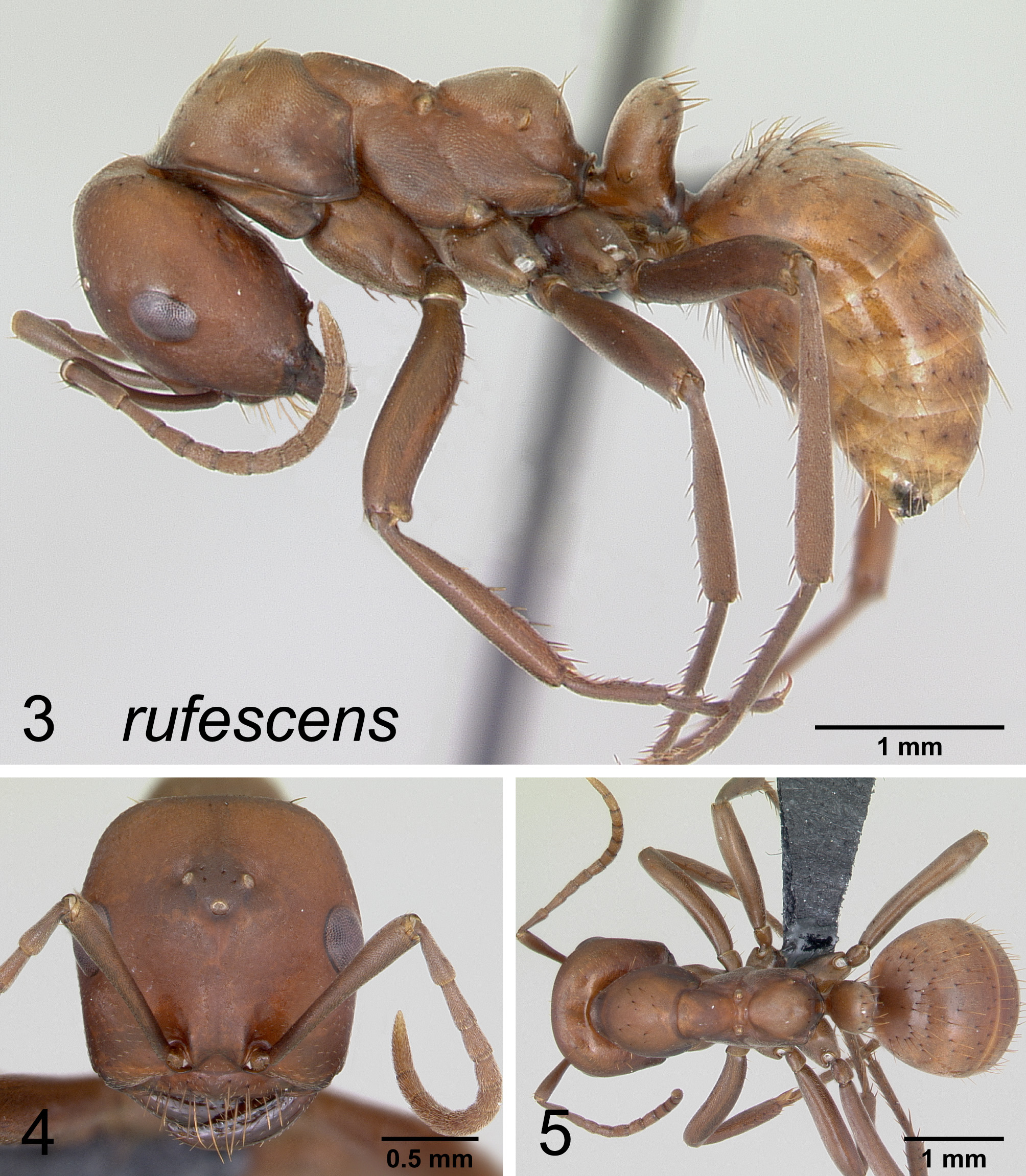
Figures 3, 4, 5 View FIGURES 3 – 5
Formica rufescens Latreille 1798: 44 . Syntype worker, gyne: FRANCE [MNHN] (not examined).
Polyergus rufescens: Latreille 1804:179 . Schenck, 1852:70 (male). Forel, 1874:137 (gynandromorph). André, 1882:163 (redescription of worker, gyne, male).
Polyergus testacea Fabricius 1804: 400 . Holotype gyne: “ CZECHOSLOVAKIA ” (= CZECH REPUBLIC) [ZMUC] (not examined). Smith, 1858:57 (synonymy).
Polyergus rufescens tianschanicus Kuznetsov-Ugamsky 1927: 41 . Syntype worker, gyne, male: KYRGYZSTAN, Issik-Kul, Tal des Dzhulek, Tian-Shan Mountains [ZIN] (not examined). New Synonymy.
Measurements (N=41) HL 1.23–1.72 (1.59), HW 1.20–1.66 (1.51), SL 0.99–1.31 (1.22), ½ VeM 0–11 (2.41), ½ PnM 3–13 (6.8), WL 1.96–2.60 (2.37), GL 1.44–2.18 (2.13), HFL 1.36–1.92 (1.78), CI 90–99 (95), SI 77–88 (81), HFI 110–130 (1.18), FSI 136–155 (146), LI 3.23–4.32 (3.96), TL 4.67–6.98 (6.14).
Worker description. Head subrectangular, its length greater than breadth; with conspicuous vertex pilosity (usually 6–12 setae) on most specimens from more western locations and no vertex pilosity (0–1 seta) on specimens from farther east; scape apex reaching about 1/4 the distance between eye and vertex corner, weakly clavate in the apical third, or gradually thickening apically; pronotum with (6)10–20 (25) erect setae; mesonotum with profile flat or very weakly convex for most of its length; propodeum evenly rounded; petiole high, its profile about equal in height to propodeum, petiole straight-sided, petiolar dorsum convex, not emarginate or weakly emarginate; first tergite densely pubescent, with numerous, bent or strongly flexuous, decumbent pilosity concentrated in anterior half of sclerite.
Head matte; mesonotum matte; gaster matte.
Color deep red (especially west) to orangey red (especially east) with weak to notable infuscation (deep, often purplish tinted, brown) of pleura, gaster and appendages in darker individuals.
This is the unique Polyergus species of Europe and western temperate Asia, and appears morphologically to be closely related to the breviceps group, particularly the essentially Mexican species topoffi . Future genetic study should deepen our understanding of the relationships of this apparent outlier of what is otherwise a western North American group, distinguished from all other red Polyergus by its Eurasian distribution. It is most similar to topoffi among the American species, differing by its slightly (average) narrower head and petiole, and denser, more regular array of bent or strongly flexuous, decumbent pilosity on the first tergite.
Specimens from western and especially southwestern Europe are darker in color than those from Asia. Populations of central western Europe have a more pilose vertex than those from the Iberian Peninsula, northern and eastern Europe, and central Asia (½ VeM usually 2–12, compared to ½ VeM 0–2). Lighter color and reduced pilosity were noted as characteristic of the subspecies tianschianicus in Kuznetsov-Ugamsky’s (1927) description, but in fact, color variation seems to be a west to east clinal feature, and the reduced pilosity is a characteristic of peripheral populations, as it is also found in samples from the far west of the range. Thus, the two traits do not covary. In any case, the subspecies are indistinguishable by any other ecological, metric or obvious morphological characters; hence, my synonymy of this subspecies.
Etymology. Latreille coined this name from the Latin verbal form “ rufescens ”, meaning reddish or fading to red.
Natural history. Found from Atlantic western Europe east to mountains of western China and “Central Asia”. Extending to 57o N and 88o E, then south to the Caspian, Black, and Mediterranean Coasts.
The “classical” summaries of the behavior of this ant are from Huber (1810) and Wheeler (1910). In the last decade or so, a number of papers, especially those by le Moli’s Laboratory in Italy, have refined our knowledge of this species, particularly regarding the role of secondary compounds in regulating their behavior (Castracani et al. 2003, 2005, 2008; Grasso et al. 2003, 2004, 2005; Le Moli et al. 2001; Romani et al. 2006; Visicchio et al. 2001, 2003, 2007). The following natural history is paraphrased from a summary kindly provided by Bernhard Seifert (pers. comm., 2009) “ P. rufescens is characteristic of dry, semi-dry and sparse grasslands of any sort that supports sufficiently dense host populations. Hosts vary geographically and include a variety of species: F. cunicularia (16 observations), F. fusca (12), F. rufibarbis (10), F. clara (3), F. gagates (3) and F. cinerea (1). Local host species preferences are obvious, and considering the whole distributional range, host species selection appears to be a trade-off between host species abundance and mortality risk—strong and aggressive colonies of F. clara and F. cinerea are only attacked in the absence of less resistant alternatives. In many regions of Central Asia, F. c l a r a is a main host, as it is a dominant species there and has smaller workers than in Central Europe.” And from Roland Schultz (pers. comm., 2012) “The host of P. rufescens in cases from Kyrgyzstan, Kazakhstan and western China is F. clara . Formica clara is the most common “ Serviformica ” in the high steppes of the Tianshan Mountains of China and Kyrgyzstan, at the altitudes in which also Polyergus appears, below 2500 m. In one case, Seifert found 5 workers of F. exsecta , including one freshly eclosed from the pupa, among a lot of F. clara and P. rufescens .” In a sample from the Tarbagatai Mountains Kazakhstan, Schulz confirmed a mix of F. clara and F. rufibarbis as hosts. In addition, I have series from the Pyrenees with F. gerardi , where this is the most abundant potential host. Seifert’s abundance/trade-off hypothesis seems plausible and testable; there is much opportunity for careful study of host selection in this species (as also in the quite polylectic North American P. mexicanus ).
Distribution of studied specimens. BULGARIA (Locality?) Her J. Morįk (JCTC); FRANCE (Dépt.?) Smith coll. Pres. By Mrs. Farren White 1899-303 (LACM); FRANCE BOUCHES du RHÔNE Marseilles. Lawn at Ctr. Nat. Rech. Sci. 2 Sept. 1986 L. Morel (JCTC); FRANCE SEINE-et-MARNE Fontain Bleau VI-59 (JCTC); GERMANY BAVARIA Würzburg VII-17-07 WM Wheeler (LACM); HOLLAND Roermond. VII-25-47 JKA van Boven (LACM); ITALY VENETO Verona. Settimo Coll. Priv. C. Baroni 17-9-56 (JCTC); REPUBLIC of SERBIA Yugoslavia, Serbia. Ratje, Zupa 13.VIII.1993. I Petrov (LACM); SLOVAKIA Czechslovakia, Slovakia May, 1984 P. Werner #358 (JCTC); SLOVAKIA Trebišov District. Somotor 5.7.72. K Denea (LACM); SPAIN CÁCERES V-85 (JCTC); SPAIN CATALUNYA El Corredor, Barcelona 10/Jul/1987 X. Espadaler #1 (JCTC); SPAIN CUENCA 30-V-81 (JCTC); SWITZERLAND TICINO Locarno VII-4-07 WM Wheeler (LACM); SWITZERLAND VAUD 1902-120. Col. Bingham (LACM); SWITZERLAND VAUD June-7, 1907 W.M.W. (LACM); SWITZERLAND VAUD Swisse. La Sarraz 6.6.49 (LACM); SWITZERLAND VAUD La Sarraz. 26.7.49. Bibikoff (LACM); SWITZERLAND: VAUD 8-VIII? Col. Bingham (LACM).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.