Myxobolus opsaridiumi, Lekeufack-Folefack, Guy Benoit, Tchoutezo-Tiwa, Armandine Estelle, Fomena, Abraham & Mansour, Lamjed, 2021
|
publication ID |
https://doi.org/ 10.5852/ejt.2021.733.1221 |
|
publication LSID |
lsid:zoobank.org:pub:901649C0-64B5-44B5-84C6-F89A695ECEAF |
|
DOI |
https://doi.org/10.5281/zenodo.5706205 |
|
persistent identifier |
https://treatment.plazi.org/id/03DB87FE-4711-DD26-DF41-FD9CFC59936A |
|
treatment provided by |
Plazi |
|
scientific name |
Myxobolus opsaridiumi |
| status |
sp. nov. |
Myxobolus opsaridiumi sp. nov.
urn:lsid:zoobank.org:act:F3105EFD-A1BC-483F-8FC8-5D07FC9B82F0
Figs 1–4 View Fig View Fig View Fig View Fig ; Table 1 View Table 1
Etymology
The specific epithet is related to the host genus name.
Type material
CAMEROON • infected skin, muscle and spleen of Opsaridium ubangiensis with plasmodia; Centre Region , Anga River, Yaounde ; deposited in parasitological collection of the Zoology Department Museum, College of Science, King Saud University, Saudi Arabia; Myxospsar/12/2018 .
Taxonomic summary
Type host
Opsaridium ubangiense Pellegrin, 1901 (Cyprinidae) .
Infected tissues
Skin, muscles and spleen.
Prevalence
54.7% (288 parasitized fish out of 526 examined).
Vegetative stages
Ovoid, spherical or ellipsoid plasmodia, variable in size, measuring from 0.3 mm to 2.5 mm in length and 0.2 mm to 1.5 mm in width.
Description of myxospores ( Fig. 1 View Fig )
Mature spores were ovoid to subspherical in frontal view and lenticular in lateral view ( Fig. 1 View Fig A–B). The valves were relatively thick, without edge markings. Intercapsular processes were absent. The spore size was 10.7 ± 0.14 (10–11.5) µm long, 9 ±0.15 (8–10) μm wide and 6.2± 0.7 (5.6–7.2) μm thick. The two ovoid polar capsules were equal in size, converging and opening together at the anterior end of the same pore ( Fig. 1A View Fig , C–D). They measured 5± 0.07 (4.3–6.0) μm in length and 2.7± 0.07 (2.2–3.0) μm in width. Polar filaments were coiled from 5 to 7 turns perpendicular to the longitudinal axis of the polar capsules ( Fig. 1D View Fig ). A sporoplasm containing an iodinophilous vacuole of varying shape and size filled the entire space below the polar capsules ( Fig. 1A View Fig ).
Clinical finding and histopathology
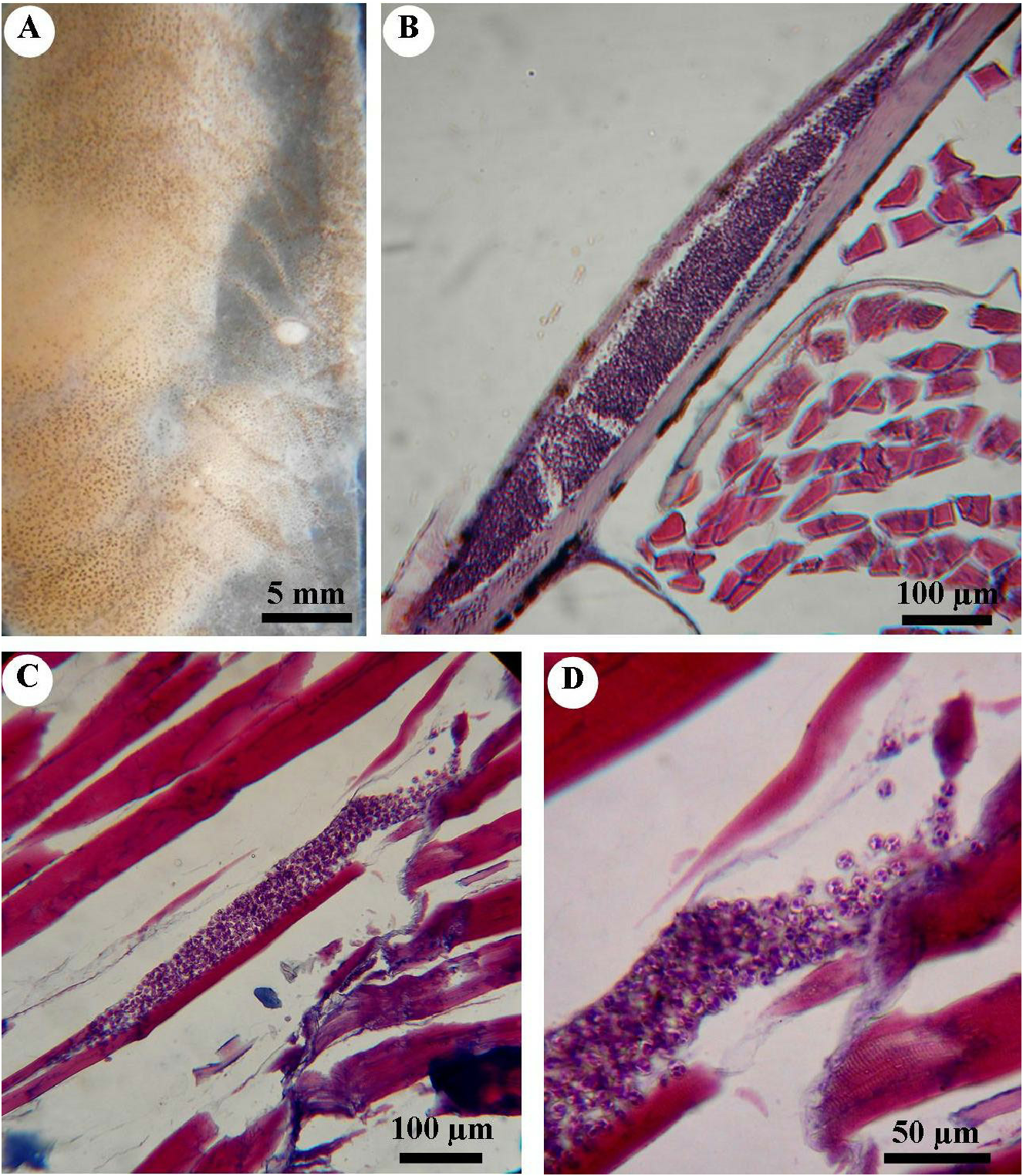
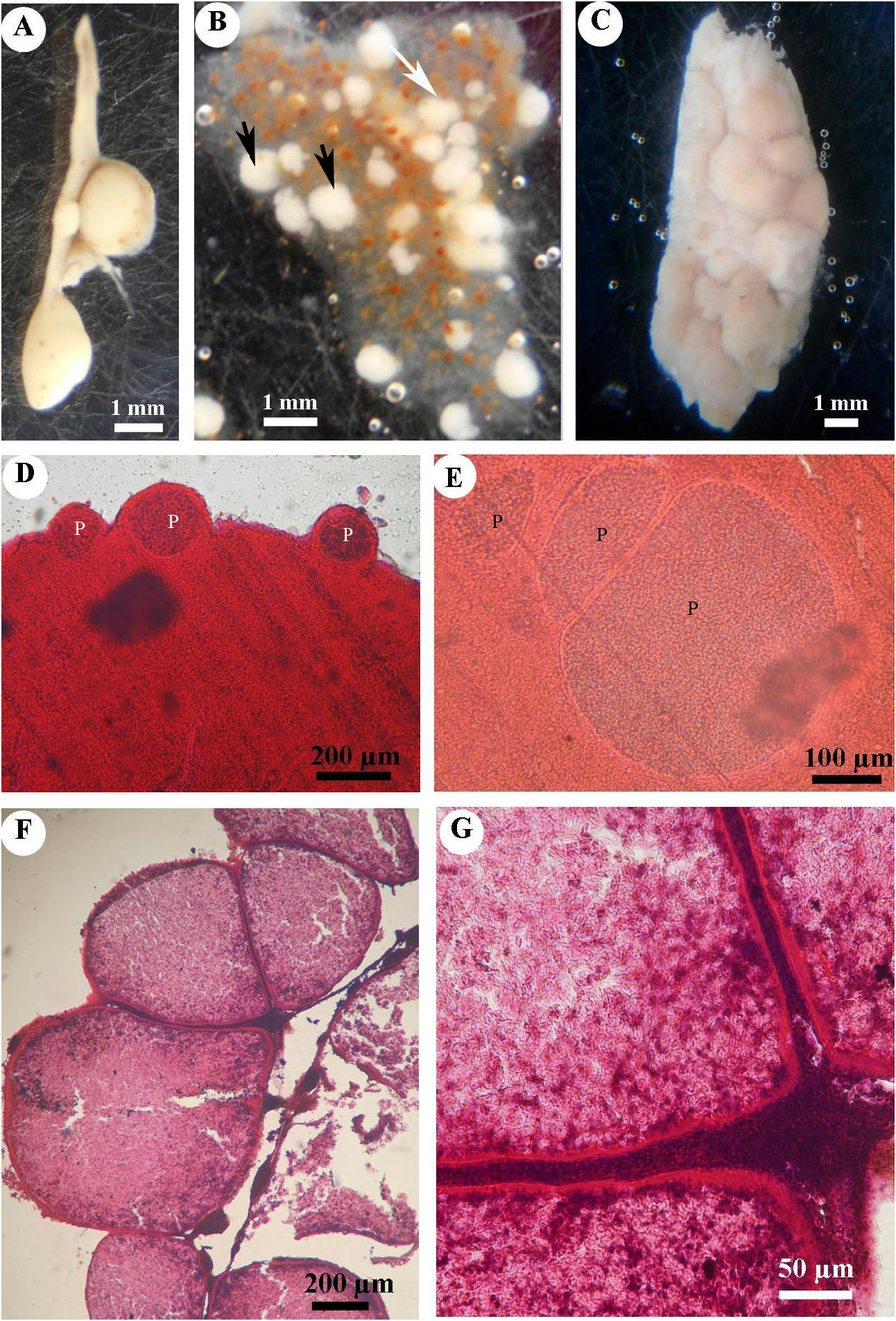
Based solely on gross observation of the fish, no signs of disease were observed. Parasitized fish harbored cysts on skin, muscles and spleen. On skin, white cysts up to 2 mm long were collected from the body flanks of some fish ( Fig. 2A View Fig ). Sections revealed that plasmodia developed in the connective tissue of the dermis beneath the underside of scales ( Fig. 2B View Fig ). Plasmodia were flattened and surrounded by a thin membrane and an internal endoplasm comprising a loosely defined matrix containing developed spores ( Fig. 2B View Fig ).
Some plasmodia were spotted within muscle cells ( Fig. 2C View Fig ). Plasmodia were spindle-shaped, centrally located in the cell and not surrounded by a visible wall. No evidence of inflammation or immune-cell recruitment was seen. The integrity of myofibrils within the infected fibers showed some degree of lysis, with partial loss of myofibrillar details and striations ( Fig. 2 View Fig C–D). These lesions were observed close to plasmodia. Mature spores were scattered in the cytoplasm of infected cells ( Fig. 2D View Fig ).
Infected spleens had plasmodia of up to 2.5 × 1.5 mm ( Fig. 3A View Fig ). They were white, isolated or clustered ( Fig. 3B View Fig ). Some infected spleens were heavily infected and plasmodia were randomly distributed in the whole organ. In these cases, abnormal enlargement of the spleen was evident ( Fig. 3C View Fig ). Histological sections revealed that, for moderately infected spleens, cysts were either fixed to the external region of the organ ( Fig. 3D View Fig ) or completely implanted within it ( Fig. 3E View Fig ). Development of cysts in the spleen was asynchronous ( Fig. 3 View Fig D–F). Atrophy of the adjacent splenic cells surrounding the cyst was likely due to mechanical compression ( Fig. 3 View Fig F–G). Each plasmodium was surrounded by a wall of a monolayer of flat cells ( Fig. 3 View Fig F–G). The central part of the plasmodium was occupied by fully mature spores, with initial stages of development visible in the periphery ( Fig. 3G View Fig ).
Phylogenetic position
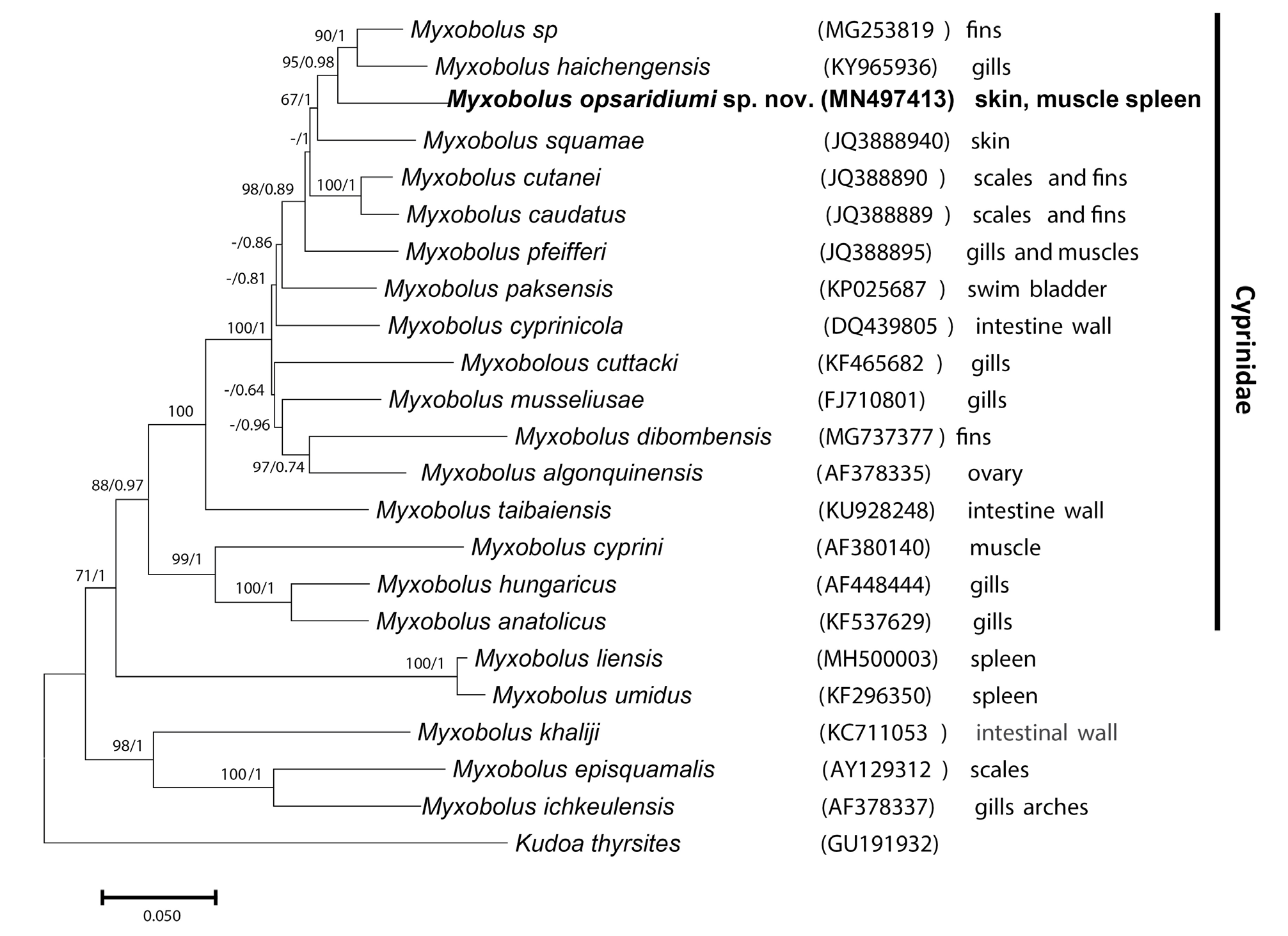
Partial SSU rDNA sequences obtained fromdifferent organs were 100% identical.The consensus sequence of 1667 base pairs was submitted to GenBank with the accession number MN497413 View Materials . This sequence did not match any publicly available myxozoan sequence. The sequence with the highest nucleotide similarity, at 91.8%, was for Myxobolus haichengensis Chen, 1958 (GenBank entry KY965936 View Materials ), which reportedly infects the gills of Abbottina rivularis (Basilewsky, 1855) . Similarity with M. dibombensis Folefack et al., 2019 , a species we recently sequenced from Labeobarbus batesii (Boulenger, 1903) in Cameroon, was only 88.6%.
The phylogenetic position of the newly sequenced species was analyzed with maximum likelihood and Bayesian inference methods. Both methods produced an identical topology. Myxobolus opsaridiumi sp. nov. occurs in a large clade that includes species infecting cyprinids ( Fig. 4 View Fig ). The new species exhibits the highest phylogenetic affinity with M. haichengensis , Myxobolus sp. (accession number MG253819 View Materials ) from the fins of Capoeta tinca (Heckel, 1843) off Anatolia, and M. squamae Keysselitz, 1908 infecting the skin of the common barbel Barbus barbus (Linnaeus, 1758) .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Variisporina |
|
Family |
|
|
Genus |