Nescicroa Karny, 1923
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5073.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:AA3269D1-CA2F-4528-BC9D-3A4C75D05BD9 |
|
DOI |
https://doi.org/10.5281/zenodo.14198363 |
|
persistent identifier |
https://treatment.plazi.org/id/03DB87EE-FF69-9DCB-FF40-5EFDFD12F534 |
|
treatment provided by |
Plazi |
|
scientific name |
Nescicroa Karny, 1923 |
| status |
|
Genus Nescicroa Karny, 1923 View in CoL rev. stat.
( Figs. 61A & C View FIGURE 61 , 62 View FIGURE 62 , 63 View FIGURE 63 )
Type-species: Necroscia terminalis Redtenbacher, 1908: 561 , pl. 26: 6, by original designation.
Günther, 1929: 629.
Bradley & Galil, 1977: 182.
Hennemann, 1998: 121 (in part).
Otte & Brock, 2005: 224 (in part).
Paranecroscia, Seow-Choen, 2016: 181 (in part). [Erroneous synonym]
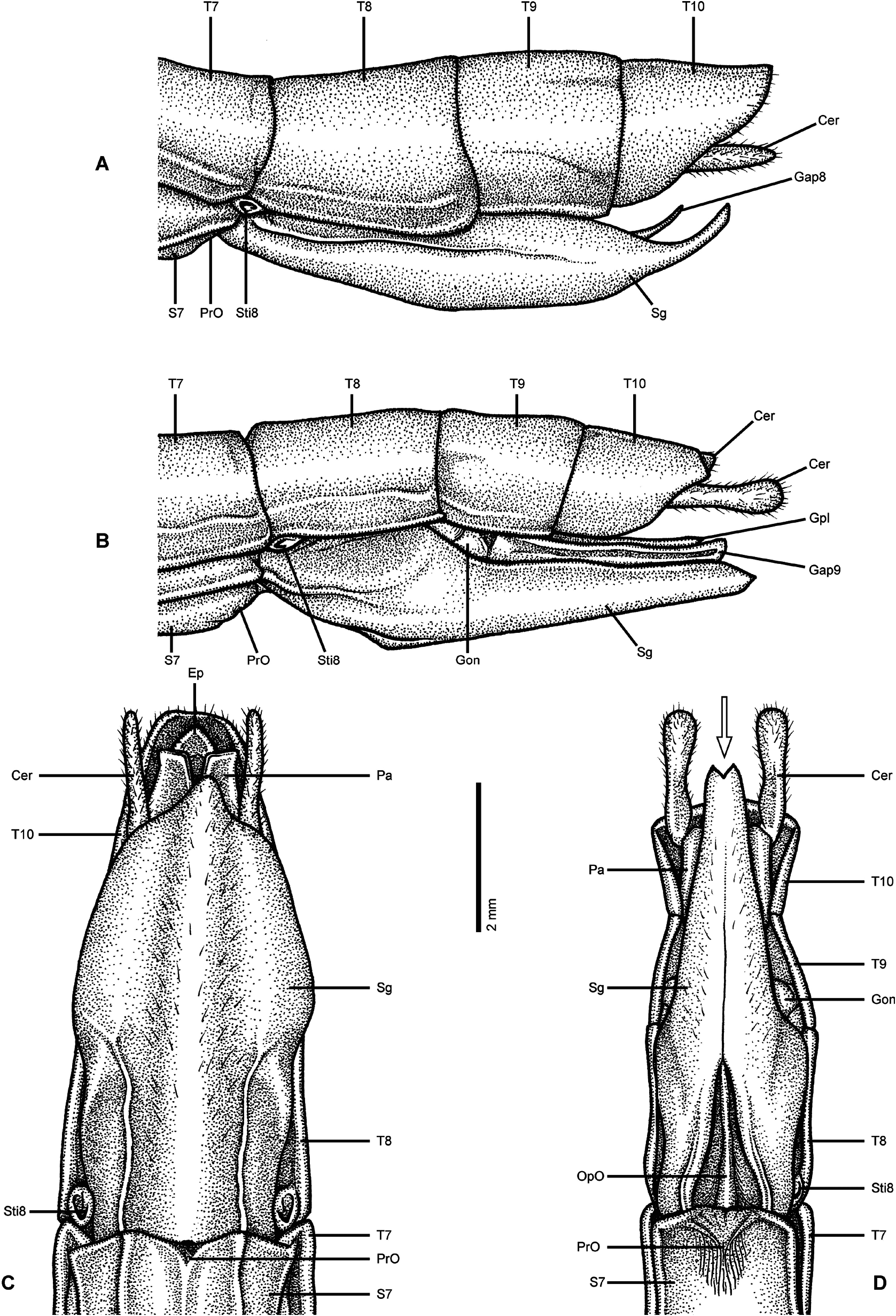
Diagnosis: ♀, ♂. Small to medium-sized (body length <80.0 mm), fairly stocky Necrosciinae with well developed alae in both sexes that at least reach to abdominal segment VI. Sexual dimorphism distinct with ♂♂ much smaller and more slender than ♀♀; also colouration may be very different between the sexes. Often colourful and ± multicoloured insects; anal fan of alae uniform in colour and ranging from hyaline over translucent grey to dark pink. Head sub-spherical to squarish and hardly longer than wide, smooth; no ocelli. Antennae shorter than body (♀♀) or ± as long as body (♂♂); filiform. Mesothorax moderately slender and in ♂♂ rarely with a slight swelling subanteriorly; <3x longer than pronotum. Mesonotum with ± distinct, obtuse medio-longitudinal carina and smooth to minutely granulose. Mesosternum smooth but with a fine medio-longitudinal carina. Tegmina squamiform, rather flattened and posterior margin obtusely angular. Terminalia of ♀♀: Anal segment roughly triangular in dorsal aspect with apex ± protruded and the posterior margin rounded to obtusely pointed; covering epiproct, which is not visible in dorsal aspect ( Fig. 61C View FIGURE 61 ). Cerci cylindrical straight and ± reaching to apex of anal segment. Gonapophysis VIII with apex narrowed and slightly elongated, fully concealed in subgenital plate ( Fig. 61A View FIGURE 61 ). Gonoplacs very small. Subgenital plate broad, weakly scoop-shaped and with a narrowed, often ± up-curving apex ( Figs. 61A, C View FIGURE 61 ); at beast reaching to tip of anal segment. Terminalia of ♂♂: Tergum IX not distinctly longer than VIII or X. Anal segment with a ± distinct posteromedian indention or excavation; the outer angles ± swollen and occasionally with a very few minute teeth interiorly. Epiproct scale-like and visible in dorsal aspect; sometimes projecting slightly beyond tip of anal segment. Cerci variable in size and at best projecting beyond tip of abdomen by the length of anal segment; shape slender and ± club-like with apex obtusely swollen. Vomer small with a single and mostly straight terminal hook. Poculum variable in size, mug-shaped with the posterior margin entire and at best reaching 1/3 along anal segment. Legs slender, completely unarmed and with all carinae rather obtuse and indistinct; tibiae may be almost round in cross-section. Profemora weakly compressed and curved basally. Medioventral carina of femora very obscure. Probasitarsi ± as long as following three tarsomeres combined, meso- and metabasitarsi less than combined length of tarsomeres II and III. Eggs ovate to almost spherical or barrel-shaped.
Differentiation. Similar and supposedly closely related to Singaporoidea Seow-Choen, 2017 and Neonescicroa Seow-Choen, 2016 . Whereas a distinction from the latter genus is not possible at this juncture (see comment on Neonescicroa below), Nescicroa may be distinguished from Singaporoidea by: the much stockier shape and relatively shorter body segments with the mesonotum no more than 3x the length of the pronotum; generally much more complex colouration and usually being multi-colourous (general colour mostly green or brown in Singaporoidea ); shorter and broader, more squarish head; shorter and broader apically truncate tegmina; notably less carinated legs (tibiae may be almost round in cross-section) and less compressed and just weakly curved base of the profemora.
From Paranecroscia , with which this genus was synonymised in error, Nescicroa can readily be separated by the morphology of the terminalia of ♀♀ ( Fig. 61 View FIGURE 61 ), by lacking a secondary ovipositor and having a simple, broad and scoop-shaped subgenital plate ( Figs. 61A, C View FIGURE 61 ), slender and straight cerci and with the small epiproct fully concealed by the ± protruded apex of the basically triangular anal segment ( Fig 61C View FIGURE 61 ; see detailed explanation below). In association with the ♀♀ genital morphology the eggs differ by being spherical, ovate or barrel-shaped. A reliable distinction between ♂♂ appears difficult.
Comments: Nescicroa Karny, 1923 was established without a formal description and originally only contained its type-species N. terminalis (Redtenbacher, 1908) . Numerous species have subsequently been attributed to the genus by various authors, but a definition of the Nescicroa has never been undertaken. Seow-Choen (2016: 181, 210) synonymised Karny’s genus with Paranecroscia Redtenbacher, 1908 (Type-species: P. operculata Redtenbacher, 1908: 210 ) solely for the fact that the type-species of both genera “ are similar in many aspects ”, but failed to provide any morphological characters that would justify this synonymy. Moreover, Seow-Choen (2016: 181) stated that Nescicroa as assembled previously has been polyphyletic. Unfortunately, the very sketchy new definition of Paranecroscia presented by Seow-Choen does also not give any morphological characters that would be useful for a generic distinction within the speciose subfamily Necrosciinae . Instead, the only trait of definitive character mentioned “ The female operculum (= subgenital plate) boat-shaped with pointed, notched or upcurved tip ” describes two fundamentally different character stades that suggests polyphyly of Paranecroscia as newly defined by Seow-Choen (2016: 181, 210). Actually, detailed examinations of the terminalia of ♀♀ of the type-species of Nescicroa and Paranecroscia ( Fig. 61 View FIGURE 61 ) prove this assumption and clearly show the synonymy introduced by Seow-Choen (2016) to be wrong. Thus, Nescicroa is here re-established as a valid genus (rev. stat.). A new diagnosis of Nescicroa is presented above and the justification for removing the genus from synonymy with Paranecroscia is as follows:
The type-species of Paranecroscia and Nescicroa exhibit two fundamentally different types of genitalia in ♀♀ ( Fig. 61 View FIGURE 61 ), which are associated with two fundamentally different egg morphologies and oviposition strate-gies. In Paranecroscia there is a secondary ovipositor whereas this is lacking in Nescicroa . In Paranecroscia the subgenital plate is narrow, groove-like with the lower surface ± straight in lateral aspect (fig. 61B), elongate and usually reaches to or slightly projects over the apex of the abdomen. The apex is notched and bifid ( Fig. 61D View FIGURE 61 ). The gonapophyses IX are prominently enlarged and mostly melted with the smaller gonapohyses VIII with both ± reaching to the tip of the subgenital plate ( Fig. 61B View FIGURE 61 ). The gonoplacs are free, elongated, filiform and just slightly shorter than the gonapophyses. A distinct gonangulum is visible between abdominal tergum IX and the subgenital plate ( Fig. 61B View FIGURE 61 ). The cerci are conspicuously enlarged, projecect distinctly beyond the apex of the abdomen and have the apex strongly swollen and club-like. The anal segment (= tergum X) has the posterior margin indented or excavated medially to give space to a scale-shaped epiproct that is well visible in dorsal aspect ( Fig. 61B View FIGURE 61 ). The secondary ovipositor formed by the subgenital plate, gonapophyses and gonoplacs is used for depositing eggs in substrates like bark, moss, lichens or soil. The eggs of Paranecroscia -species are very elongate, bullet-like or okra-shaped, notably curved in lateral aspect and have the posterior end with two distinct longitudinal carinae that converge to an acutely pointed tip. The micropylar plate is elongate and lanceolate in shape. All these characters of Paranecroscia are shared with e.g. Necroscia Serville, 1838 and Orthonecroscia Kirby, 1904 and suggest close relation to these two genera. In strict contrast, no such secondary ovipositor is seen in N. terminalis , the type-species of Nescicroa , and all other species here re-transferred to the genus. The subgenital plate is of a simple shape, being broad, scoopshaped with a narrowed and acute, ± up-curving tip and rarely reaches the apex of the abdomen ( Figs. 61A, C View FIGURE 61 ). Nor the gonapophyses, neither the gonoplacs are notably enlarged and all three pairs of genital valves are almost fully concealed by the subgenital plate ( Fig. 61A View FIGURE 61 ). The cerci are simple, straight and cylindrical. The anal segment is basically triangular in dorsal aspect and has the apex ± protruded and rounded to acutely pointed. This protruded apex of the anal segment overlaps the very small epiproct, which is fully hidden under the anal segment and not visible in dorsal aspect ( Fig. 61C View FIGURE 61 ). The eggs are spherical, ovate or barrel-shaped, have a small oval micropylar plate and are simply dropped by the ♀♀. All these characters of the ♀♀ terminalia are shared with Neonescicroa Seow-Choen, 2016 and Singaporoidea Seow-Choen, 2017 and suggest close relation to these to genera rather than Paranecroscia . Hence, the overall similarity in general shape and colour of certain species of Nescicroa and Paranecroscia , noted by Seow Choen (2016: 181, 210) can be revealed as nothing but convergent evolution, which is very commonly observed within Phasmatodea .
The status of Neonescicroa Seow-Choen, 2016 (type-species: Necroscia excelsa Redtenbacher, 1908 ) is questionable and deserves scrutiny. The genus agrees with Nescicroa in most aspects including the morphology of the ♀♀ terminalia and might be polyphyletic in the present composition.
The species P. albilateralis Hennemann, 1998 , P. inconspicua (Redtenbacher, 1908) , P. macra (Redtenbacher, 1908) , P. poeciloptera (Rehn, 1904) and P. tenella (Günther, 1935) are not congeneric and are here transferred to Singaporoidea Seow-Choen, 2017 (see below).
Distribution. Sumatra, Java, Borneo, Sulawesi, Moluccas, New Guinea & Solomon Islands.
Species included:
The following list of species inludes all species that can be confirmed as members of Nescicroa Karny, 1923 . Several further species may belong in Nescicroa but could either not be examined for this study or are only known from single holotypes, which lack the terminalia that are indespensable for a confirmed decision on their generic affiliation.
1. Nescicroa angustata (Redtenbacher, 1908: 562) . rev. comb.
Distribution: Borneo.
2. Nescicroa compacta (Redtenbacher, 1908: 562) . rev. comb.
Distribution: Java & Sumatra.
3. Nescicroa contracta (Redtenbacher, 1908: 562) . rev. comb.
Distribution: Sumatra.
4. Nescicroa heinrichi Günther, 1935a: 25 , pl. 2: 17. rev. comb.
Distribution: Sulawesi.
5. Nescicroa obliterata (Redtenbacher, 1908: 561) . rev. comb.
Distribution: New Guinea.
6. Nescicroa papuana (Brancsik, 1898: 65, pl. 2a–b). rev. comb.
Distribution: New Guinea.
7. Nescicroa rammei ( Günther, 1935a: 26) .
Distribution: Sulawesi.
8. Nescicroa redempta (Redtenbacher, 1908: 562) . rev. comb.
Distribution: Java.
9. Nescicroa resignata (Redtenbacher, 1908: 560) . rev. comb.
Distribution: New Guinea.
10. Nescicroa rivalis (Redtenbacher, 1908: 562) . rev. comb.
Distribution: Borneo.
11. Nescicroa rufescens ( Hennemann, 1998: 109, pl. 4: 7–8, figs. 14–15). rev. comb., n. stat.
Distribution: Sulawesi.
12. Nescicroa ruficeps ( Kirby, 1904: 437) . n. comb.
Distribution: Solomon Islands (Guadalcanal).
13. Nescicroa sanguinata (Redtenbacher, 1908: 560) . rev. comb.
Distribution: New Guinea.
14. Nescicroa smaragdula ( Bates, 1865: 357, pl. 45: 7). rev. comb.
= Necroscia albofasciata Redtenbacher, 1908: 560 . n. syn. *
= Necroscia graminea ( Bates, 1865: 356) . n. syn. *
Distribution: Moluccas (Halmahera & Bacan), New Guinea.
15. Nescicroa splendida n. sp.
Distribution: Sulawesi.
16. Nescicroa tereticollis (Redtenbacher, 1908: 561) . rev. comb.
Distribution: New Guinea.
17. Nescicroa terminalis (Redtenbacher, 1908: 561, pl. 27: 6). rev. comb.
Distribution: Borneo.
18. Nescicroa tumescens (Redtenbacher, 1908: 560) . rev. comb. **
Distribution: Moluccas
19. Nescicroa viridilineata ( Bates, 1865: 352) . rev. comb.
= Necroscia frontalis Redtenbacher, 1908: 522 . n. syn. ***
Distribution: Moluccas (Seram, Ambon, Buru).
* Examination of the holotypes of Bates N. smaragdula and N. graminea , both from the island of Bacan, and taking the very similar colour pattern and difference between the sexes of N. splendida n. sp. into account leaves no doubt that N. graminea is the opposite sex and ♀ of N. smaragdula . Thus, it is here synonymised (n. syn.). The holotype of Redtenbacher’s N. albofasciata in ZMAS, and also from the island of Bacan, is a typical ♀ of this species and therefore also synonymised (n. syn.).
** The type series of Redtenbacher’s N. tumescens comprises two distinct species. The specimens from Bacan ‘Batjan’ in the collection of NHMW are N. smaragdula ( Bates, 1865) .
*** Necroscia frontalis was described from a total of seven specimens from the islands of Ambon and Buru in the collections of NHMW, MNHU and one from Java in SMTD. The latter locality stated for the ♀ in SMTD however is doubtful. Exami-nation of Redtenbacher’s type specimens and comparison with the ♀ and ♂ of N. viridileata Bates, 1865 from Seram in OXUM leave no doubt they are the same species. To provide stability to the species described by Bates , the ♀ in OXUM is here selected as the lectotype of N. viridilineata . Redtenbacher’s frontalis is synonymised with the ♀ from Amboina with the left wing opened and a blue collection number label stating ‘1657’ attached to it designated as the lectotype (n. syn.). A further ♂ from Seram is contained in the author’s collection and matches very well with the paralectotype of viridilineata .
Keys to the Sulawesian species of Nescicroa View in CoL
♀♀
1. Subgenital plate short, not notably projecting over posterior margin of abdominal tergum IX.......................... 2
- Subgenital plate longer, reaching at least half way along anal segment........................................... 3
2. Head, thorax and apex of femora and tibiae red ( Figs. 62A, C View FIGURE 62 )............................................ rufescens View in CoL
- Head, thorax dark green; apex of femora and tibiae dark yellow........................................... rammei View in CoL
3. Mesonotum 2.6x longer than pronotum; head reddish laterally; tegmina and costal region of alae with anterior margin distinctly black; anal region of alae translucent grey............................................................ heinrichi View in CoL
- Mesonotum only 2x longer than pronotum; head plain green with a yellow postocular streak on genae ( Fig. 63D View FIGURE 63 ); tegmina and costal region of alae without a black anterior margin; anal fan of alae translucent pink ( Fig. 63B View FIGURE 63 ).......... splendida View in CoL n. sp.
♂♂ *
1. Mesonotum> 2.5x longer than pronotum, slender; epiproct projecting beyond tip of anal segment..................... 2
- Mesonotum only 2.1x longer than pronotum, slightly swollen anteriorly; epiproct very small, transverse and not reaching to posterolateral angles of anal segment ( Fig. 63J View FIGURE 63 )................................................. splendida View in CoL n. sp.
2. Head, thorax and apex of alae red; epiproct almost semicircular and hardly projecting beyond posterolateral angles of anl segment ( Fig. 63G View FIGURE 63 )................................................................................ rufescens View in CoL
- Head and thorax dark green, apex of femora dark yellow; epiproct triangular and projecting strongly beyond tip of anal segment.......................................................................................... rammei View in CoL
* ♂♂ of N. heinrichi Günther, 1935 View in CoL are not known.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Nescicroa Karny, 1923
| Hennemann, Frank H. 2021 |
Paranecroscia, Seow-Choen, 2016: 181
| Seow-Choen, F. 2016: 181 |
Necroscia graminea ( Bates, 1865: 356 )
| Bates, H. W. 1865: 356 |