Ditomyia sohnsi Fitzgerald, 2020
|
publication ID |
https://doi.org/10.11646/zootaxa.4859.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:19608730-D202-4863-AF29-742F9B791732 |
|
DOI |
https://doi.org/10.5281/zenodo.4412977 |
|
persistent identifier |
https://treatment.plazi.org/id/03DB7A34-520C-FFB5-FF58-79DB2E89F422 |
|
treatment provided by |
Plazi |
|
scientific name |
Ditomyia sohnsi Fitzgerald |
| status |
sp. nov. |
Ditomyia sohnsi Fitzgerald View in CoL n. sp.
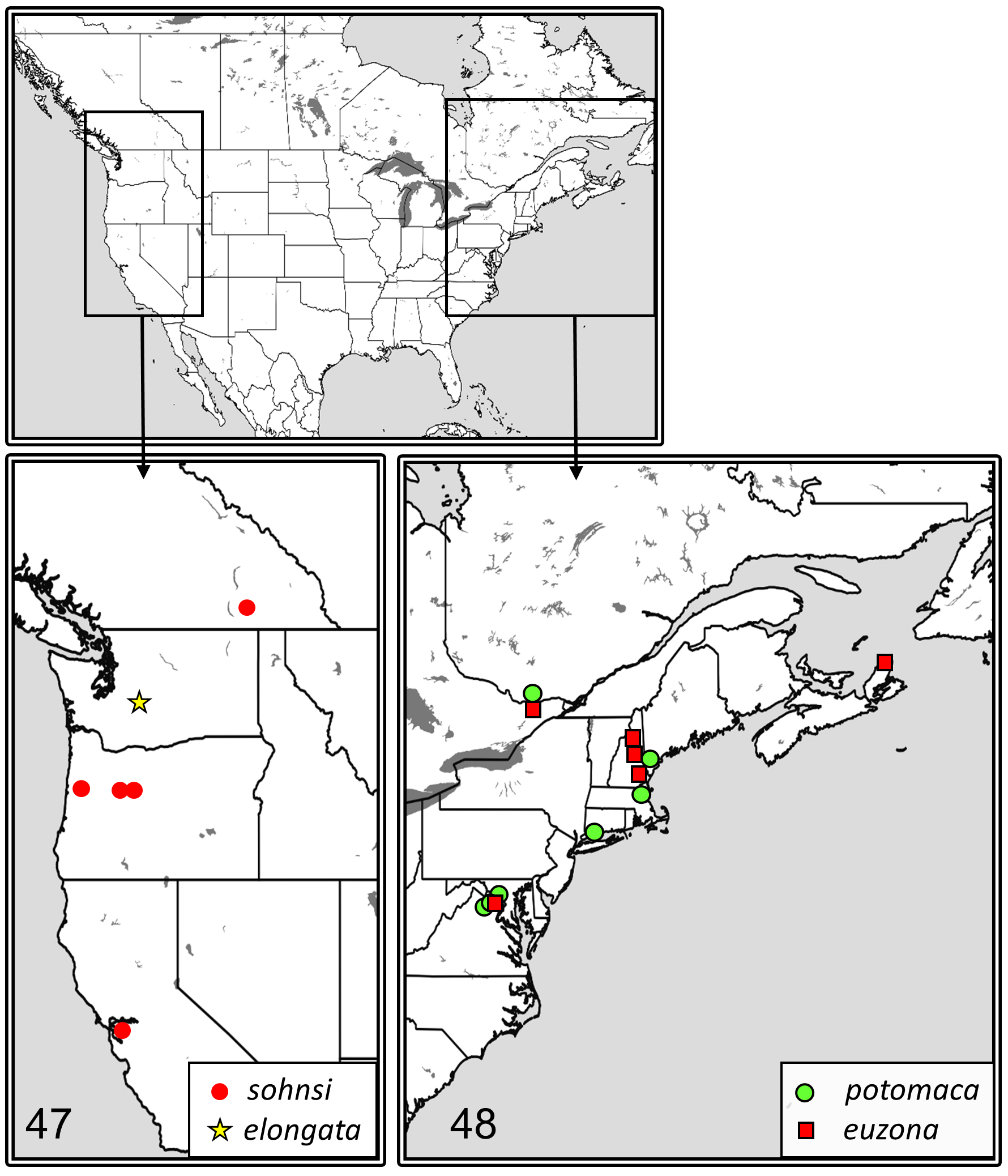
Figs. 4 View FIGURES 1–4 , 6 View FIGURES 5–6 , 24–28 View FIGURE 24 View FIGURES 25–28 , 47 View FIGURES 47–48 (Map)
urn:lsid:zoobank.org:act:E521678F-326A-4761-AB6C-DB00D3C62B7D
Type Material. Holotype: Male, point-pinned ( CNCI), USA: OREGON: Benton Co., 6.4 mi. up Woods Creek Road from jct. Hwy 20, MT across old road, fir/alder/maple, 14 July–13 Aug. 2014, S. Fitzgerald, MT 019 . Paratypes: CANADA: BRITISH COLUMBIA: Lemon Creek , 117’16”, 40’30”, 31 July 1967, logged, J.H. Shepard , 1 female ( CNCI); same except, 24 August 1967, virgin , 1 female ( CNCI) ; USA: CALIFORNIA: Alameda Co., Chabot Park, Castro Valley , 27 Feb. 2017, reared from polypore fungus, Joyce Gross, BG #1345034 , 1 female ( CSCA); OR- EGON: same as holotype , 2 females ( SFC); same as holotype except 24 June–14 July 2014, MT 018 , 1 female ( SFC); same as holotype except 13 Oct.–12 Nov. 2014, MT 027 , 1 female ( SFC); same as holotype except 5 June–11 July 2015, MT045 , 1 female ( SFC); Benton Co., Marys Peak nr. N. ridge trailhead, ca. 44.530104 -123.547080, 9 Sept.–3 Oct. 2014, S. Fitzgerald, MT025 GoogleMaps along big log, 2 females (1 SFC, 1 OSAC); Linn Co., Hackleman Creek , 0.6 mi. E Tombstone Pass, 44.397501, -122.131401, 1 Aug.–22 Sept. 2016, S. Fitzgerald, MT 056 GoogleMaps , 1 male ( SFC); Jefferson Co., Black Butte, SW Black Butte Road , ca 2.3 mi. W Rd. 11, ca. 44.415312, -121.639624, 19 April–12 July 2017, S. Fitzgerald, MT 064 GoogleMaps , 1 male ( SFC) .
Diagnosis. Ditomyia sohnsi can be distinguished from other Nearctic Ditomyia by the following combination of characters: Wings hyaline with macrotrichia evenly distributed in cell br+bm ( Fig. 4 View FIGURES 1–4 ), antennal flagellomeres greyish-brown, contrasting with pale yellow scape and pedicel ( Fig. 6 View FIGURES 5–6 ); scutum pale yellow with three light brown stripes ( Fig. 24 View FIGURE 24 ); male terminalia as Figs. 25–27 View FIGURES 25–28 , female terminalia as Fig. 28 View FIGURES 25–28 with ultimate segment of cerci broadly rounded; female fore tibia ~1.1 times as long as fore basitarsus.
Remarks. The male terminalia of this species are very different from other Nearctic taxa, particularly the bilobate gonostylus and the gonocoxites extending posteriorly beyond the gonostylar socket as a long slender lobe. The tower-like aedeagal complex of this species is similar to that found in the Palearctic species D. claripennis Saigusa, 1973 and D. macroptera Winnertz, 1852 and at least the latter species has a distinctly bilobate gonostylus ( Saigusa 1973a, Kurina & Chandler 2018).
Etymology. The specific epithet, sohnsi , is in honor and memory of my good friend Tony Sohns (August 21, 1977 – February 6, 2019) ( Figs. 29–35 View FIGURES 29–35 ). Tony was one of my favorite people. Aside from being a great human being he was a consummate naturalist, in the sense of the old-school naturalists, with an astoundingly broad and encyclopedic knowledge of natural history. He also loved rarities, which is why it feels appropriate that this rarely collected fly bears his name. Tony was an avid collector and not just of insects; his friends all know the stories about the dead beaver in the bathtub or the emu in the freezer...he made us all laugh and inspired us with his infectious enthusiasm for biology. One of the things I loved about Tony was his perpetual willingness, at the drop of a hat, to head into the field (with or without clear objectives) and I have great memories of numerous excursions we took; such as the middle-of-the-night, middle-of-the-winter, middle-of-a-snowstorm trip to the top of a mountain to collect undescribed grylloblattids, or the road trip to eastern Washington to hunt for the unknown immature stages of Bibiodes Coquillett (Bibionidae) , or the memorable old-growth trip when we found the pupa of what would later eclose and be designated as the holotype of the rare western Nearctic axymyiid Protaxymyia thuja Fitzgerald & Wood. It saddens me that we will never share those kinds of trips again, but it feels somehow fitting, that this fly bearing Tony’s name will be out there flitting through the dappled-sunlight of the moss-covered ancient Oregon forests that Tony loved to explore. More information about Tony’s life and legacy can be found in his obituary at: https://obituaries. bangordailynews.com/obituary/anthony-sohns-1072630898.
Description. Male. Body length (including antennae): 6.0–7.0 [6.0] mm, (n=3). Head. Brown with short pale setae. Three dorsomedial ocelli, arranged in a transverse line, median ocellus much smaller. Compound eye oval with fine sparse hairs, widely separated dorsomedially, very slightly emarginated anteromedially at antennal base. Maxillary palps light brown, four-segmented. Fifteen flagellomeres, most about as long as broad, becoming slightly more elongate distally, ultimate flagellomere minute, subspherical. Flagellomeres grey-brown with short, black, stiff, setae and pale pubescence. Pedicel and scape pale yellow, contrasting in color to flagellomeres. Base of antennal setae often marked with a black spot. Thorax. Pale yellow with light brown to brown markings on pleura and three broad, light brown, longitudinal stripes on scutum (lateral stripes shorter, truncated anteriorly). Scutum evenly setose with the lateral and dorsocentral setae stronger, setae light brown, scutellum with seta restricted to posterior edge. Thoracic pleura bare. Legs. Coxa and femur pale yellow, tibia pale yellow basally, light brown distally, tarsi brown distally. Legs with dense appressed microtrichia. Fore tibia without macrosetae. Abundant short, black, erect macrosetae on all, but the ventral surface of hind tibia. Hind basitarsus slender, elongate, parallel-sided. Tibial spurs 1:2:2. Wing ( Fig. 4 View FIGURES 1–4 ). 4.5–6.0 [4.5] mm (n=3). Hyaline with dense micro- and macrotrichia, sometimes a slight brown cloud at base of Rs. Veins brown. C ending near wing tip just beyond R 4+5. Base of Sc strongly pigmented, pigmented portion ending abruptly near base of wing, followed by long, faint, distal portion (crease), ending free near level of base of Rs. Base of Rs and stem of radial fork subequal in length. R 2+3 long, base basal to level of medial fork. R-m short, vertical. Stem of M and base M 1 sometimes weak to hyaline. CuP reaching wing edge. Stem of M 1+2 shorter than fork. Abdomen. Brown with narrow, bright, pale yellow band on posterior edge of tergites. Terminalia ( Figs. 25–27 View FIGURES 25–28 ). Tergite nine reduced to a slender transverse band. Cerci, broad, fleshy, flap-like, apically truncate in lateral view. Gonocoxites extending posteriorly beyond attachment of gonostylus into an apically broadly rounded lobe. Gonostylus with two main lobes; outer lobe ( Fig. 27 View FIGURES 25–28 , gso) black, short, stout, with several stout apical spines, inner lobe ( Fig. 27 View FIGURES 25–28 , gsi), pale, broad, flap-like, the posterior juncture between the two lobes, best observed in posterior view, marked by a small black thumb-like lobe. Hypandrium basally bulbous, apically tapered and distally fused or in close association with apical broad plate-like portion of ejaculatory apodeme. Aedeagal complex (aedeagus, apex ejaculatory apodeme, paramere) forming an elongate, tower-like structure extending beyond the base of the gonostyli; slightly forked apically. Paramere only visible dorsally as transparent subrectangular plate wrapping laterally around base of aedeagus.
Female. ( Fig. 24 View FIGURE 24 ). Most aspects essentially as in male, except as follows. Body 7.0– 8.5 mm (n=3), wing 7.0– 7.5 mm (n=3). Cerci pale yellow, two-segmented, basal segment elongate, apical segment short oval ( Fig. 28 View FIGURES 25–28 ). Sternite 8 longitudinally divided medially.
Distribution ( Fig. 47 View FIGURES 47–48 ). Presently known from British Columbia, Canada, the Cascade and Coastal Mountains of Oregon, and central, coastal California.
Bionomics. A conservative examination of the collecting records suggests the flight period is probably at least June–October, but may begin as early as April and go into November (difficult to determine exact flight period since records are based on Malaise trap samples which included multiple months per sample). Immature stages remain undiscovered. However, the female specimen from California was reared from an unidentified hard polypore in an area with mostly oaks ( Quercus agrifolia Née ), bay trees ( Umbellularia californica (Hook. & Arn.) Nutt. ) and a few pines and it is believed the polypore came from oak; the polypore was collected 4 February and it is believed the adult emerged around 27 February (Joyce Gross, University of California, Berkeley, pers. comm. 2019). In Oregon adults were all collected via Malaise traps ( Figs. 36–37 View FIGURES 36–37 ), in forest types ranging from mature Douglas fir forest to Douglas fir forest edge mixed with maple and alder, to mixed fir and pine woods on the east side of the Cascades.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |