Dugesia naiadis, 2013
|
publication ID |
https://doi.org/10.1111/zoj.12077 |
|
persistent identifier |
https://treatment.plazi.org/id/03DB7724-E201-836C-FC32-FDD5FD5FFEEC |
|
treatment provided by |
Marcus (2021-08-27 08:11:37, last updated by Plazi 2023-11-04 23:28:03) |
|
scientific name |
Dugesia naiadis |
| status |
|
DUGESIA View in CoL POPULATIONS
We performed a GMYC approach ( Pons et al., 2006; Fontaneto et al., 2007) to compare the units delimited by this method with those identified in the morphological analysis and to detect possible cryptic species. We used the partial COI sequences of 155 individuals of Dugesia from 34 localities ( Table S1). GMYC detects the change from population processes (coalescence of alleles) to speciation and extinction processes through analysis of branching rate patterns, setting a threshold between the inter- and intraspecific relationships. To obtain the ultrametric tree necessary for this approach, we conducted a phylogenetic analysis in BEAST v1.7.3 ( Drummond & Rambaut, 2007), using a fragment of COI (745 bp) from 2–5 individuals per sampling locality ( Table S1). A lognormal relaxed clock with a substitution rate of 0.017 substitutions per lineage and per million years was applied (cf. Solà et al., 2013). The analysis was run under a GTR + I + Γ evolutionary model. Three monophyletic clades were forced: (1) Dugesia species , without D. sicula and D. naiadis (used as outgroup); (2) Dugesia species , without D. sicula , D. naiadis and Dugesia from Central Europe; (3) Dugesia species , without D. sicula Lepori, 1948 , D. naiadis Sluys sp. nov., Dugesia from Central Europe and D. cretica ( Meixner, 1928; Solà et al., 2013). Monte Carlo Markov chains were run for 150 000 000 generations, sampling every 15 000 trees. The parameters were checked to have reached an effective sampling size (ESS) value of over 100 after a 10% burn-in with Tracer v.1.5 ( Rambaut & Drummond, 2007).
The BEAST tree obtained was submitted to the SPLITS (SPecies LImits by Threshold Statistics; Ezard, Fujisawa & Barraclough, 2009) package for R (available at http://r-forge.r-project.org/projects/ splits/), which implements the GMYC approach. The program also performs likelihood ratio tests (LRTs) between (a) the null and GMYC models to test whether one or multiple species are involved, and (b) single and multiple threshold options.
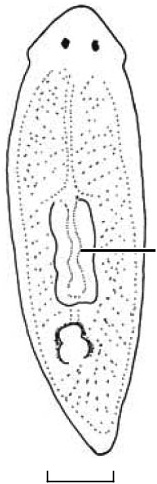
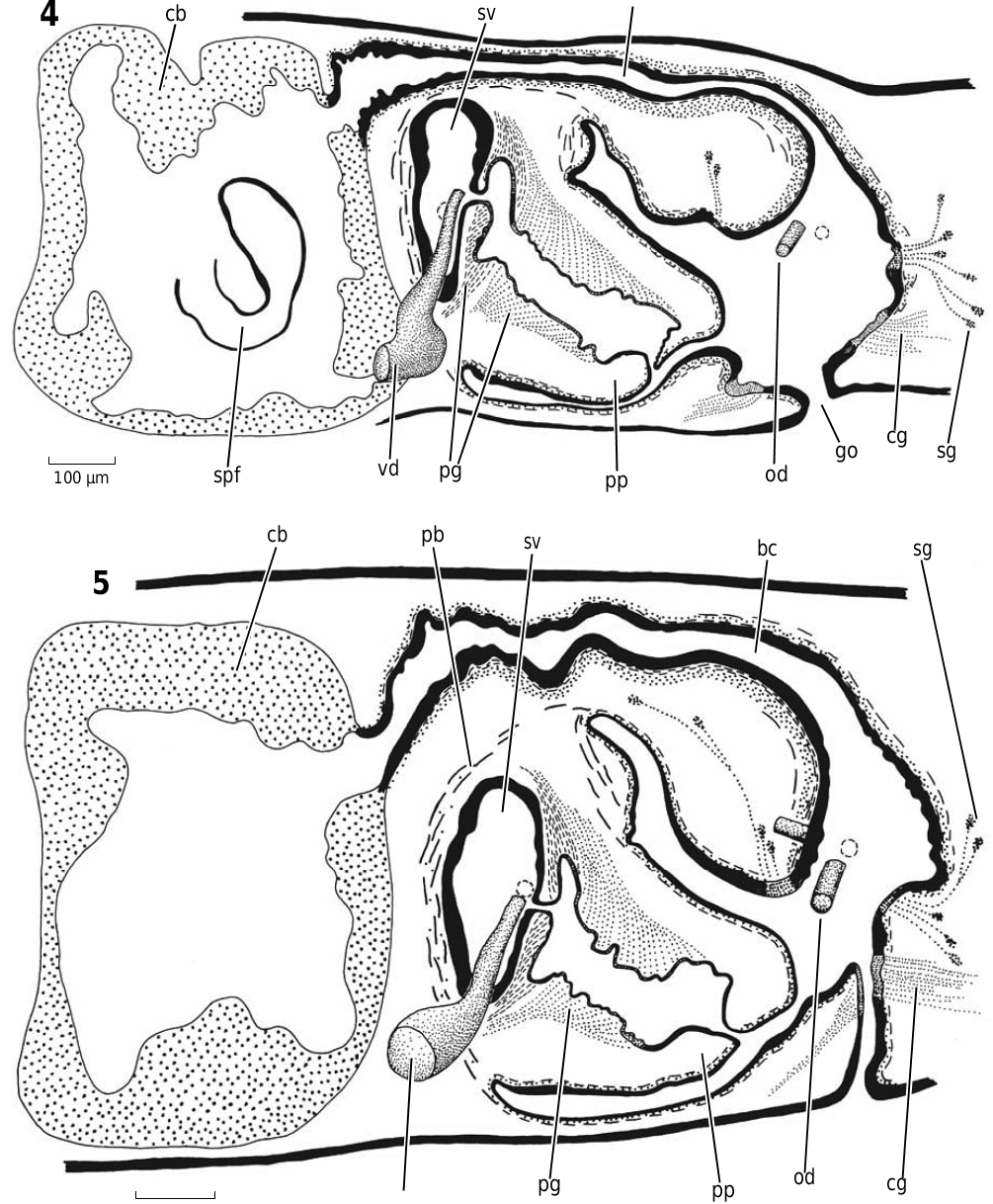
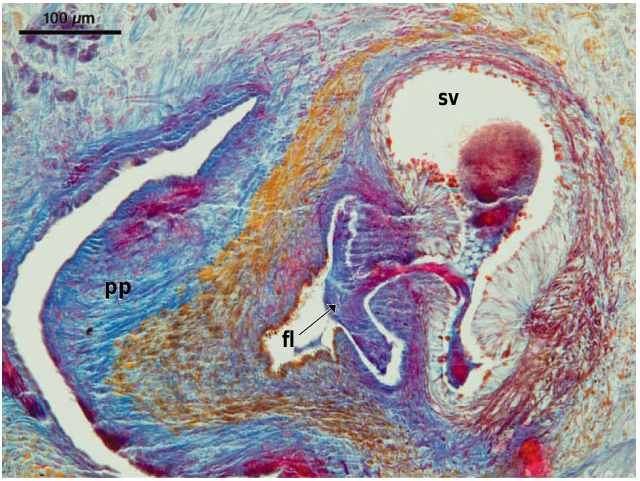
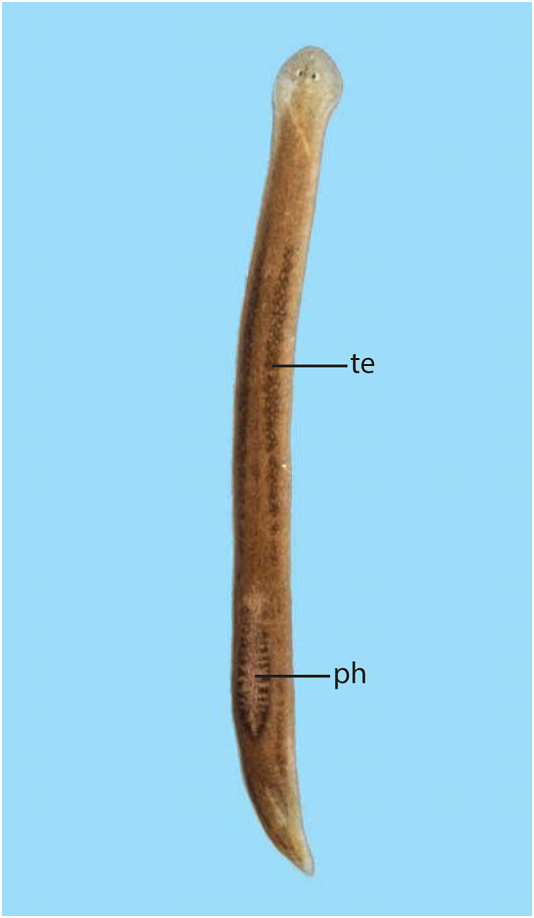
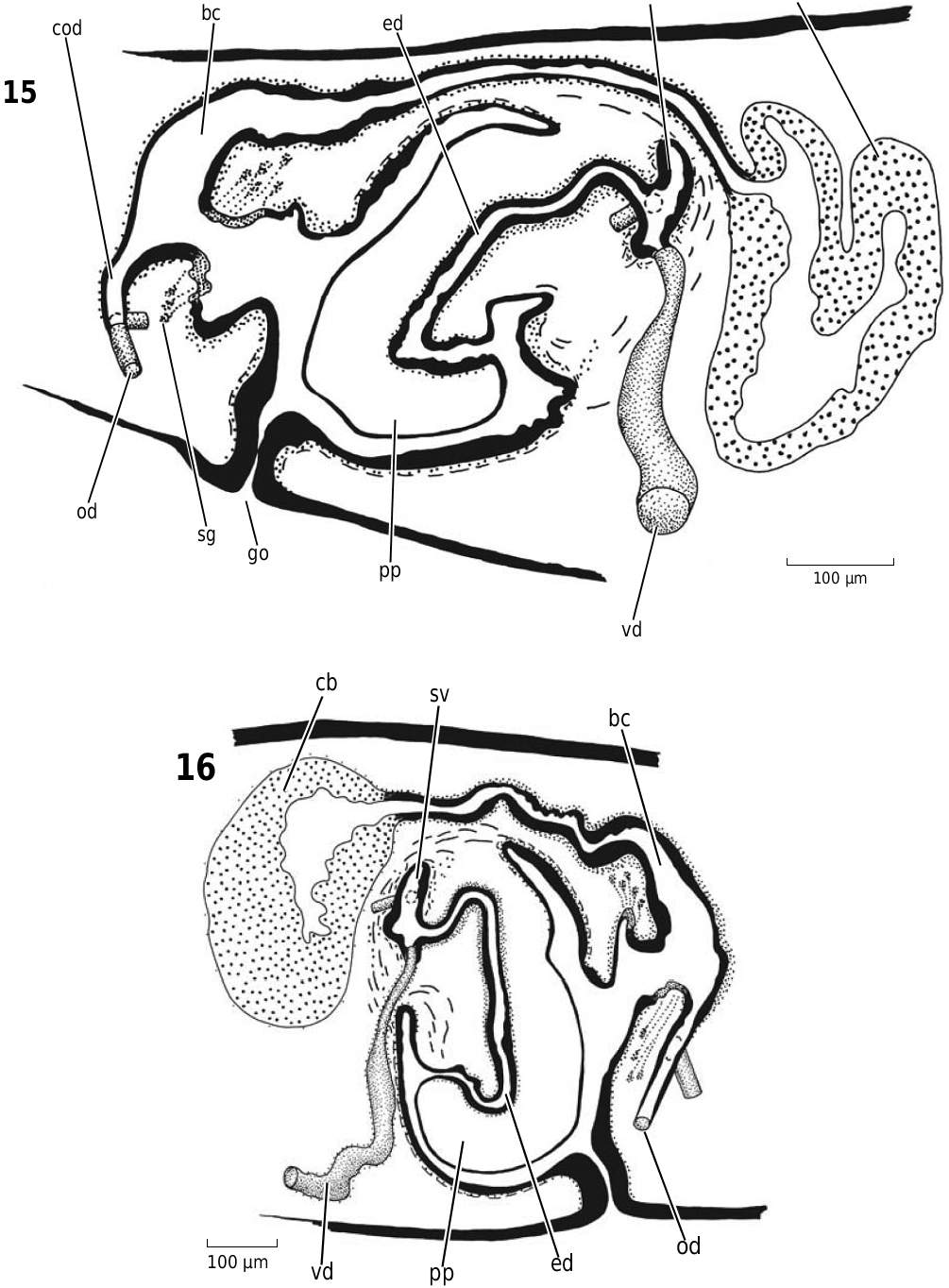
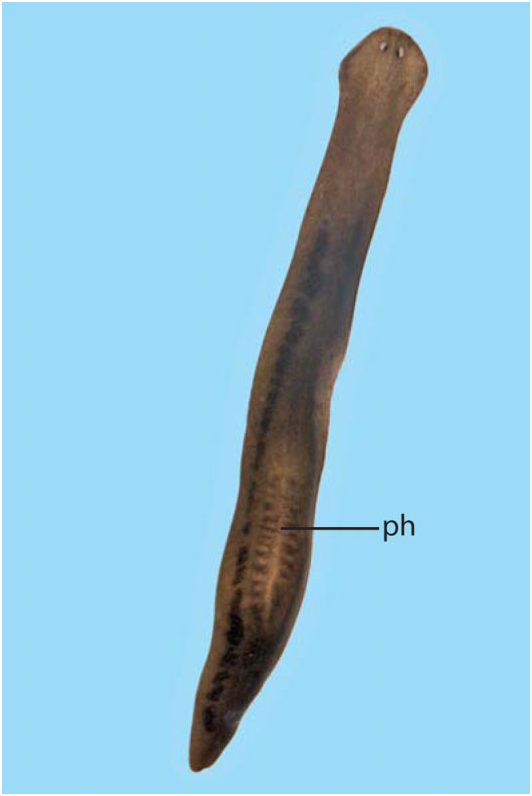
Abbreviations used in Figures 3–18 View Figure 3 View Figures 4, 5 View Figure 6 View Figures 7, 8 View Figure 9 View Figure 10 View Figures 11, 12 View Figure 13 View Figure 14 View Figures 15, 16 View Figure 17 View Figure 18 : bc, bursal canal; cb, copulatory bursa; cg, cement glands; cod, common oviduct; cs, cyanophilic secretion; dpf, dorsal penial fold; ed, ejaculatory duct; fl, flap; go, gonopore; in, intestine; od, oviduct; pg, penial glands; ph, pharynx; pp, penis papilla; sg, shell gland; spf, spermatphore; sv, seminal vesicle; te, testis; vd, vas deferens.
Drummond A, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7: 214.
Ezard T, Fujisawa T, Barraclough T. 2009. Splits: species' limits by threshold statistics. R package version 1.0. Available at: http: // R-Forge. R-project. org / projects / splits /
Fontaneto D, Herniou EA, Boschetti C, Caprioli M, Melone G, Ricci C, Barraclough TG. 2007. Independently evolving species in asexual bdelloid rotifers. PLoS Biology 5: e 87.
Lepori NG. 1948. Descrizione di Dugesia sicula, nuova sp. di Triclade d'acqua dolce dei dintorni di Catania. Archivio Zoologico Italia 33: 461 - 472.
Meixner J. 1928. Der Genitalapparat der Tricladen und seine Beziehungen zu ihrer allgemeinen Morphologie, Phylogenie, Okologie und Verbreitung. Zeitschrift fur Morphologie und Okologie der Tiere 11: 570 - 612.
Pons J, Barraclough TG, Gomez-Zurita J, Cardoso A, Duran DP, Hazell S, Kamoun S, Sumlin WD, Vogler AP. 2006. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Systematic Biology 55: 595 - 609.
Sola E, Sluys R, Gritzalis K, Riutort M. 2013. Fluvial basin history in the northeastern Mediterranean Region underlies dispersal and speciation patterns in the genus Dugesia (Platyhelminthes, Tricladida, Dugesiidae). Molecular Phylogenetics and Evolution 66: 877 - 888.
Figures 4, 5. Dugesia effusa Sluys sp. nov. 4. ZMA V.Pl. 7114.2. Sagittal reconstruction of the copulatory apparatus. 5. ZMA V.Pl. 7114.1. Sagittal reconstruction of the copulatory apparatus.
Figures 7, 8. Dugesia improvisa Sluys & Solà sp. nov. 7. ZMA V.Pl. 7116.2. Sagittal reconstruction of the copulatory apparatus. 8. ZMA V.Pl. 7116.1. Sagittal reconstruction of the copulatory apparatus.
Figure 9. Dugesia improvisa Sluys & Solà sp. nov. Photomicrograph of penial complex of specimen ZMA V.Pl. 7116.4, showing the sickle-shaped flap of tissue or secretion.
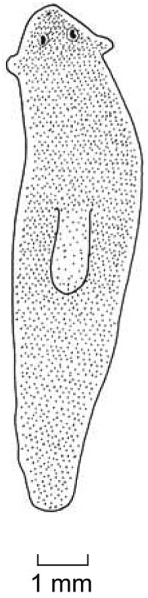
Figures 11, 12. Dugesia naiadis Sluys sp. nov. 11. ZMA V.Pl. 7117.2. Sagittal reconstruction of the copulatory apparatus. 12. ZMA V.Pl. 7117.1. Sagittal reconstruction of the copulatory apparatus.
Figure 13. Dugesia parasagitta Sluys & Solà sp. nov. Photomicrograph of large dorsal penial fold in specimen ZMA V.Pl. 7118.1.
Figure 14. Recurva postrema Sluys & Solà sp. nov. Photograph of external features (scale bar not available).
Figures 15, 16. Recurva postrema Sluys & Solà sp. nov. 15. ZMA V.Pl. 7122.4. Sagittal reconstruction of the copulatory apparatus. 16. ZMA V.Pl. 7122.1. Sagittal reconstruction of the copulatory apparatus.
Figure 17. Recurva conjuncta Sluys sp. nov. ZMA V.Pl. 7123.1. Sagittal reconstruction of the copulatory apparatus.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |