Dicyemodeca kukii, Furuya, 2018
|
publication ID |
https://doi.org/ 10.12782/specdiv.23.143 |
|
publication LSID |
lsid:zoobank.org:pub:82CD9349-810A-42F1-A602-343EBA1AE7A4 |
|
persistent identifier |
https://treatment.plazi.org/id/CB48D3B9-BD9F-4139-8633-2CB4CA9DAB0B |
|
taxon LSID |
lsid:zoobank.org:act:CB48D3B9-BD9F-4139-8633-2CB4CA9DAB0B |
|
treatment provided by |
Felipe |
|
scientific name |
Dicyemodeca kukii |
| status |
sp. nov. |
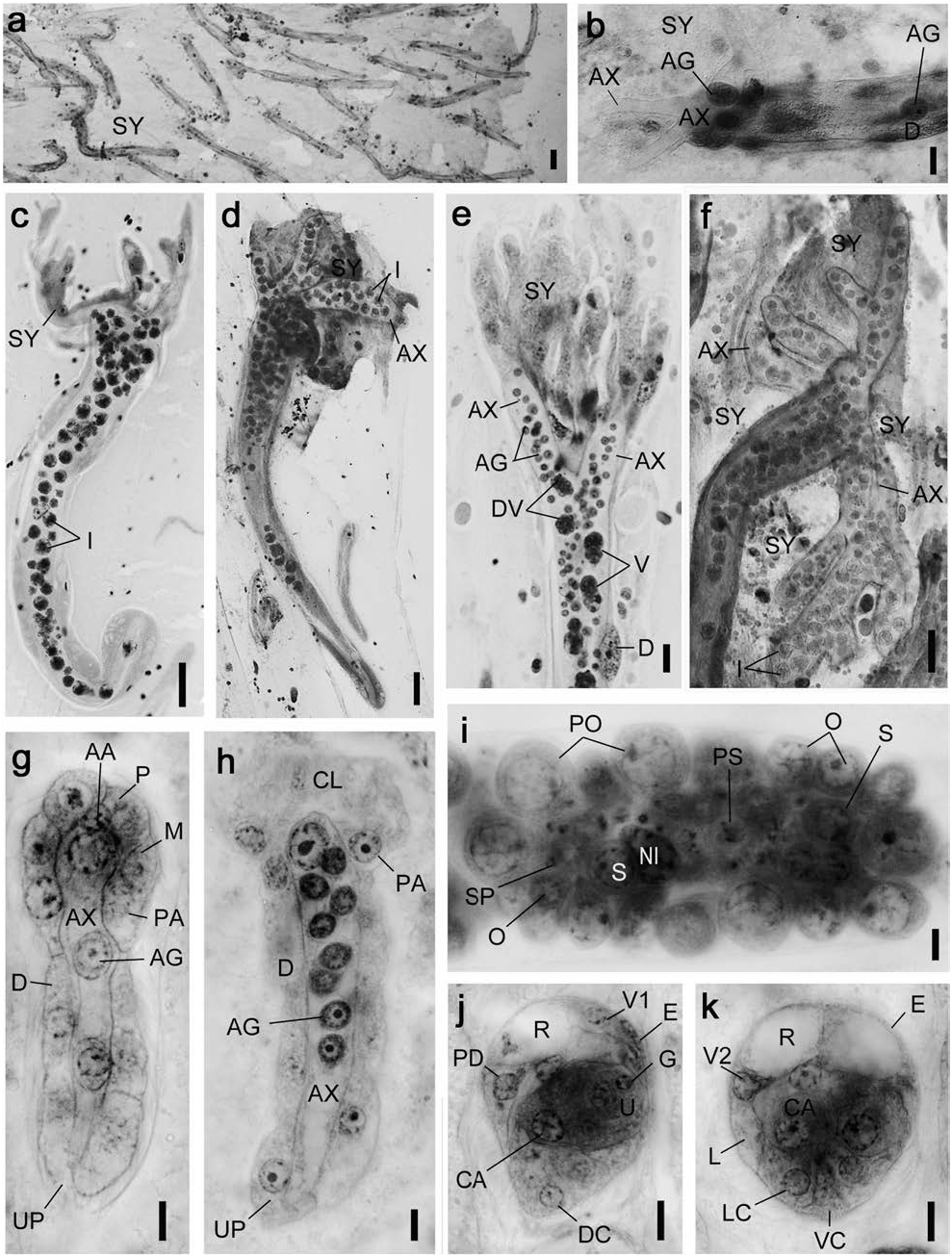
Dicyemodeca kukii sp. nov. ( Figs 22 View Fig , 23 View Fig ; Tables 2, 3)
Diagnosis. Medium-sized dicyemid; body length to 2,500 µm. Calotte cap-shaped. Vermiform stages with 24 peripheral cells; 4 propolars+6 metapolars+4 parapolars+10 trunk cells. Infusoriform embryos with 35 cells; refringent bodies liquid; and 2 nuclei present in each urn cell.
Description. Nematogens ( Figs 22a–c View Fig , 23a, c, d View Fig ). Body length 500–2,500 µm and width 50–132 µm; widest in region of metapolars; trunk width mostly uniform. Peripheral cell number 24 ( Table 3); 4 propolars+6 metapolars+4 parapolars+8 diapolars+2 uropolars. Calotte cap-shaped, round anteriorly; cilia on calotte about 4 µm long, oriented anteriorly. Propolar cells and their nuclei equal in size to metapolar cells and their nuclei ( Figs 22a, b View Fig , 23c, d View Fig ). Cytoplasm of parapolar cells containing small granules, more darkly stained by hematoxylin than cytoplasm of other peripheral cells. Verruciform cells absent. Axial cell cylindrical, round- ed anteriorly; cell extending forward to propolar cells ( Fig. 22c View Fig ). About 10 vermiform embryos present per axial cell of large individuals.
Vermiform embryos ( Figs 22f View Fig , 23e, f View Fig ). Full-grown vermiform embryos length 80–125 µm, width 16–22 µm. Peripheral cell number 24 ( Table 3); trunk cells arranged in opposed pairs. Anterior end of calotte rounded or bluntly pointed. Axial cell rounded anteriorly, extending to propolar cells; nucleus usually located in anterior half of axial cell. Anterior abortive axial cell absent. Axial cell of full-grown embryos with up to 13 agametes.
Rhombogens ( Figs 22e, d View Fig , 23b, g View Fig ). Body similar in length than nematogens but slightly stuggy, length 500–1,450 µm and width 70–140 µm. Peripheral cell number typically 24 ( Table 3). Calotte cap-shaped, rounded anteriorly. Verruciform cells absent. Axial cell shape and anterior extent similar to nematogens. One, rarely 2 or 3 infusorigens per axial cell of each parent individual. About 20 infusoriform embryos present per axial cell of large individuals. Accessory nuclei occasionally present in trunk cells.
Infusorigens ( Figs 22g View Fig , 23h; n View Fig =20). Mature infusorigens small-sized; composed of 7–35 (mode 15) external cells (oogonia and primary oocytes)+4–8 (mode 6) internal cells (spermatogonia, primary spermatocytes, and secondary spermatocytes)+3–6 (mode 5) spermatozoa. Mean diameter of fertilized eggs, 14.0 µm; that of spermatozoa, 2.5 µm. Axial cell round, diameter 12–20 µm.
Infusoriform embryos ( Figs 22h, i View Fig , 23i–k; n View Fig =20). Fullgrown embryos large, length 25.4±1.2 µm (mean± SD; excluding cilia); length-width-height ratio 1.0:0.90: 0.86; shape ovoid, bluntly rounded; cilia at posterior end 5 µm long. Refringent bodies present, liquid, occupying anteri- or 10% of embryo length when viewed laterally ( Fig. 23k View Fig ). Cilia projected from ventral internal cells into urn cavity ( Fig. 23k View Fig ). Capsule cells containing small granules. Mature embryos with 35 cells: 31 somatic+4 germinal cells. Somatic cells of several types present: external cells that covering large part of anterior and lateral surfaces of embryo (2 enveloping cells); external cells with cilia on external surfaces (2 pairs of dorsal cells+1 median dorsal cell+2 dorsal caudal cells+2 lateral caudal cells+1 ventral caudal cell+2 lateral cells+2 posteroventral lateral cells), external cells with refringent bodies (2 apical cells); external cells without cilia (1 couvercle cell+2 apical micro cells+2 shield cells); internal cells with cilia (2 ventral internal cells); and internal cells without cilia (2 dorsal internal cells+2 capsule cells+4 urn cells). Each urn cell containing single germinal cell and 2 nuclei ( Fig. 23k View Fig ). All somatic nuclei appearing pycnotic in mature infusoriform embryos.
Remarks. Dicyemodeca kukii sp. nov. has a similar peripheral cell number to the other congeners: D. dogieli Bogolepova, 1957 , D. deca (McConnaughy, 1957) , D. delamarei ( Nouvel, 1961) , and D. anthinocephalum Furuya, 1999 . However, D. kukii sp. nov. is distinguished from D. dogieli and D. deca by lacking solid refringent bodies within the apical cell ( Bogolepova 1957; McConnaughy 1957). In infusoriform embryos, the presence of two unique cell types, namely the anterior micro cells and shield cells, is common to D. kukii sp. nov. and D. anthinocephalum ( Furuya 1999) . However, the new species is distinguished from by the number of cells of infusoriform embryos (35 vs. 42). Dicyemodeca kukii sp. nov. differs from D. delamarei in calotte shape (cap-shaped vs. conical), and in lacking solid refringent bodies within the apical cell ( Nouvel 1961).
Etymology. The specific name kukii is referred to a famous local lord of “the Kuki clan” that controlled waters around the type locality during the Japanese Age of Civil War (Sengoku period, 1467–1615).
Taxonomic summary. Type material: a syntype slide (NSMT-Me-57) collected at 9 June 2004; additional syntypes on slide series No. OTY173 (5 slides) in the author’s collection.
Type locality: off Owase (34°01′N, 136°35′E), Mie Prefecture, Honshu , Kumano-nada Sea, North Pacific Ocean, Japan, depth 200 m GoogleMaps .
Other materials examined: slide series No. OT3282 (5 slides) collected off Kii-Nagashima (33°58′N, 136°28′E), Mie Prefecture, Honshu , Kumano-nada Sea, North Pacific Ocean, Japan, depth 300 m, 30 October 2015 in the author’s collection GoogleMaps .
Host: symbiotype, Octopus tenuicirrus ( Sasaki, 1929) (Mollusca: Cephalopoda: Octopoda ), male (mature), 82 mm ML (NSMT-Mo-85872).
Site : anterior ends (calottes) inserted into crypts of renal appendages within the renal sac.
Prevalence: in 2 of 48 specimens of hosts examined (4.2%).
Occurrence patterns of dicyemids in Octopus longispadiceus
In this study, seven new species of dicyemids were found in 504 of 510 individuals of O. longispadiceus : Dicyema cryptocephalum sp. nov., D. petalocephalum sp. nov., Dicyemennea acetabulum sp. nov., D. anteronucleatum sp. nov., D. mcconnaugheyi sp. nov., D. megalosomum sp. nov., and D. leptocephalum sp. nov. The prevalence of dicyemids in the host approached 100% ( Table 4). All seven species were never found individualy, and two or three (occasionally four or five) species were typically found together in a single renal sac or a single host individual ( Tables 4, 5). In two individuals of hosts, dicyemids were found in only one side of renal organs. There were various occurrence patterns, including instances where different dicyemid species were found in the renal appendage of either side. However, most of hosts (450 of 510, 88.2%) were infested by the single dicyemid species in both renal sacs.
The host specimens were obtained from six localities in the Sea of Japan ( Table 1) . The co-occurrence pattern of dicyemid species was varied among the localities. Three new species occurred in all localities: Dicyema cryptocephalum sp. nov., Dicyemennea anteronucleatum sp. nov., and D. leptocephalum sp. nov. All seven dicyemid species were found simultaneously in the northern region (Niigata Pref.) and the southern two regions (Shimane and Tottori Prefs. ) which more than 50 host specimens examined .
In all localities, both Dicyema cryptocephalum sp. nov. and Dicyemennea leptocephalum sp. nov. occurred on relatively high prevalence (50–80%) ( Table 1). Co-occurrence of these 2 species was most frequently found in a single renal sac and in a single host individual ( Table 4).
Occurrence patterns of dicyemids in Octopus tenuicirrus
Four new species of dicyemids ( Dicyemennea desmocephalum sp. nov., D. moritakii sp. nov., D. tobaense sp. nov., and Dicyemodeca kukii sp. nov.) were found in 48 individuals of O. tenuicirrus ( Table 7). All four species were typically found from two or three specimens of the host and never found simultaneously in a renal sac of the single specimen ( Table 7). In a single individual, dicyemids were found only in the left renal organ. Various occurrence patterns of dicyemid species were observed, including instances where different dicyemid species were found in the renal appendage of either side. However, the same occurrence pattern of dicyemid species was found in the renal sacs of both sides in 41 of 48 host individuals.
Dicyemennea moritakii sp. nov. showed prevalence with 100% and was therefore the predominant among four dicyemid species obtained from the host, followed by D. desmocephalum sp. nov. (38/48 host individuals). D. tobaense sp. nov. was found in 17 of 48 host individuals, but a smaller number of individuals was observed than for the other coexisting dicyemids in each renal sac. Dicyemodeca kukii sp. nov. is an uncommonly occurred species, which was detect- ed in only 2 of 48 individuals of the host.
Calotte types of dicyemid species in Octopus longispadiceus and O. tenuicirrus
The calotte shape of dicyemids is not only the major character for identification of species, but it also represents a mode of inhabiting the renal appendage. In general, four basic types of calotte shape are recognized ( Furuya et al. 2003a). Dicyemids with conical calottes (Type I) insert the anterior region of the body into crypts or folds in the renal appendages; those with cap-shaped (Type II) or disc-shaped calottes (Type III) attach to the broad, flat or gently rounded surfaces of the renal appendages; and dicyemids with irregular shaped bodies and calottes (Type IV) occur when more than three species coexist . According to the criterion of calotte shape, dicyemid species in O. longispadiceus and O. tenuicirrus are classified as follows: Type I, Dicyemennea anteronucleatum sp. nov., D. mcconnaugheyi sp. nov., and D. moritakii sp. nov.; Type II, Dicyema cryptocephalum sp. nov., D. petalocephalum sp. nov., and Dicyemodeca kukii sp. nov.; Type III, Dicyemennea acetabulum sp . nov. and D. megalosomum sp. nov., and D. tobaense sp. nov.; and Type IV, D. desmocephalum sp. nov. and D. leptocephalum sp. nov.
Two dicyemid species of Type IV ( D. desmocephalum sp. nov. and D. leptocephalum sp. nov.) adhere together with their expanded calottes ( Figs 14 View Fig , 18 View Fig ). A similar phenomenon occurs in adult stages of D. adminicula ( McConnaughey, 1949) ( Fig. 24 View Fig ). The branching pattern of their axial cell is the same as that of D. leptocephalum sp. nov. Vermiform embryos escape from adult bodies one by one, and juvenile vermiform larvae inhabit the renal sac independently ( Figs 14h View Fig , 18f View Fig ). The calottes form a thin expansion as the body grows and adheres to the nearby calottes of all other individuals ( Fig. 25 View Fig ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.