MONOPHLEBIDAE Signoret
|
publication ID |
https://doi.org/10.11646/zootaxa.5105.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:187B04D7-4C35-4E27-9B2D-A616BF59F380 |
|
DOI |
https://doi.org/10.5281/zenodo.6343187 |
|
persistent identifier |
https://treatment.plazi.org/id/03D687D0-FFA9-984E-19E8-C912FA2EE348 |
|
treatment provided by |
Plazi |
|
scientific name |
MONOPHLEBIDAE Signoret |
| status |
|
Family MONOPHLEBIDAE Signoret
Common names: Giant scales or monophlebids.
Background: The family Monophlebidae used to be regarded as a subfamily of the Margarodidae ( sensu lato) ( Morrison 1928). The splitting of Margarodidae sensu lato into 11 families by Hodgson and Foldi (2006), based on male morphology, resulted in the monophlebids becoming a separate family of 47 genera containing 265 described species. The family is morphologically very diverse, with four tribes recognized: Llaveiini (all New World genera), Drosichini , Iceryini and Monophlebini . Monophlebid females are large, but the cuticle is very delicate, making them very difficult to collect and slide-mount without damage. Live specimens should be killed and preserved in alcohol without any attempt to detach them from the plant material, to avoid tearing the cuticle and losing the mouthparts ( Sirisena et al. 2013).
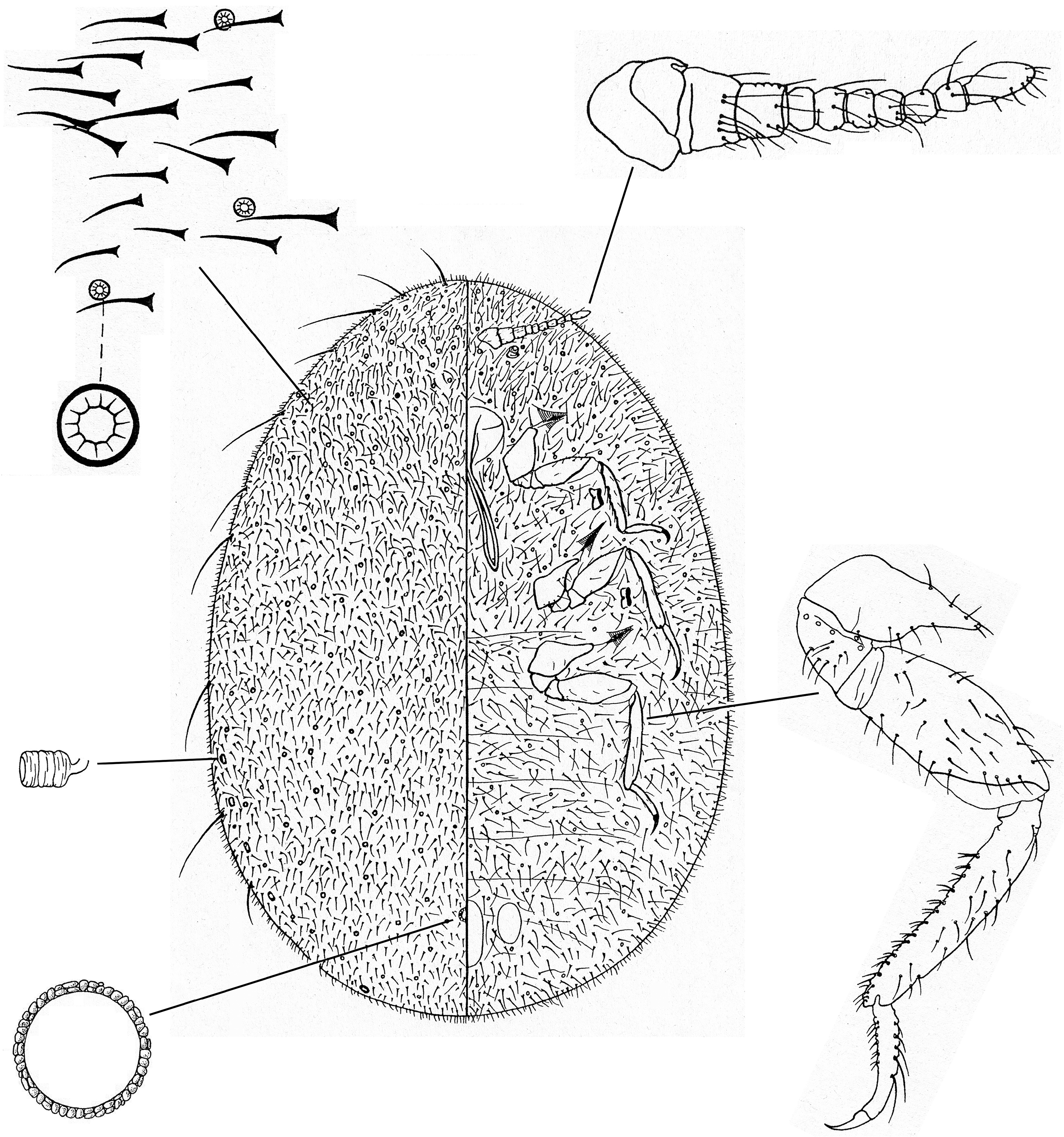
Appearance in life: Found on plant stems, branches, or leaves, often on woody hosts. Body of adult female normally oval and relatively large, up to 10 mm long or more ( Aspidoproctus maximus can reach 35 mm long); usually with white / yellow / brown wax secretions, these often flocculent, but occasionally without visible wax covering the body; sometimes with waxy projections from the body margins. To protect the eggs, some species form a large, sculptured white ovisac beneath and behind the abdomen, or develop a hollow marsupium by indentation of the venter of the abdomen. Antennae and legs well developed and heavily sclerotized, dark brown; antennae each with 9‒11 segments; legs all similar in size ( Miller et al. (2014)).
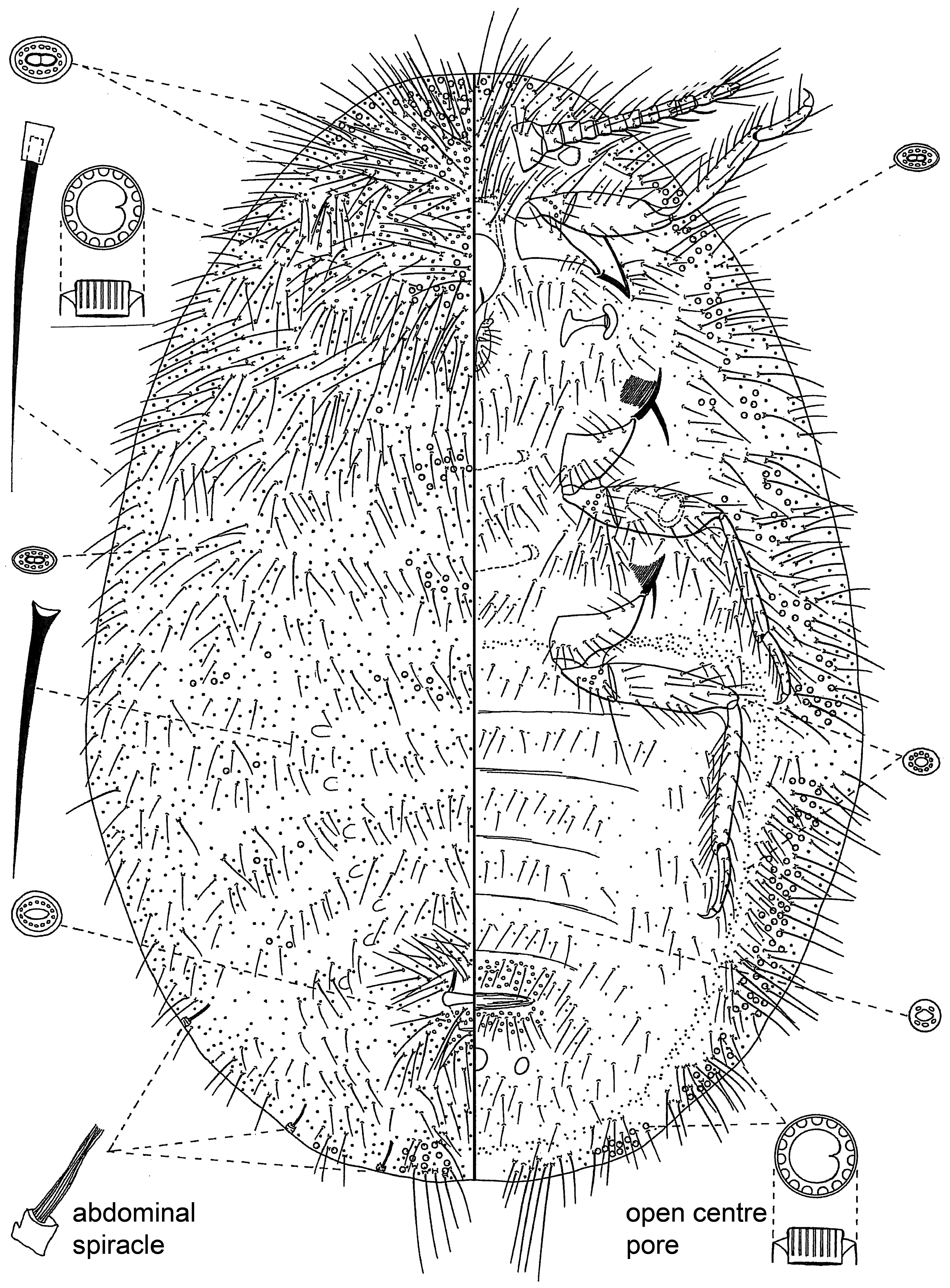
Identification: The best specimens for identification are young adult females just after the final moult, before the body has become distended with developing eggs. It is sometimes possible to identify intermediate-stage females ( Morrison (1928) provided some keys). Slide-mounted adult female up to 10 mm long or more, usually broadly oval ( Figs 9 View FIGURE 9 and 10 View FIGURE 10 ). Limbs well developed, usually conspicuous and dark; antennae each with 7‒11 segments; legs large, all similar in size, each tarsus 1 segmented; claw digitules not reaching to tip of claw and usually not enlarged apically. Internal anal tube present, the inner end with a simple sclerotized ring with or without pores. Cicatrices present. Abdominal spiracles numbering 1‒8 pairs, usually relatively conspicuous; thoracic spiracles each without pores in atrium (but pores often present just outside spiracular opening) ( Miller et al. (2014)).
Economic importance: Heavy infestations of monophlebids extract large volumes of phloem sap, drying plant tissues and causing leaf drop and branch dieback. Fouling of plant surfaces with sugary honeydew waste and the resultant sooty mould growth makes the plants unsightly and blocks light and air from the leaves, reducing photosynthesis and plant vigour, productivity and the market value of produce ( Kondo & Watson 2022 in press). The honeydew often attracts attendant ants. In India and neighboring areas, Drosicha mangiferae Green and D. stebbingii (Stebbing) (tribe Drosichini ) are pests of mango ( Karar et al. 2008). Some Icerya species are widespread pests, and in some parts of the world predators and dipteran parasites have been introduced to control them, e.g., cottony cushion scale, Icerya purchasi Maskell , is parasitised by Cryptochaetum iceryi (Williston) ( Diptera : Cryptochaetidae ), and I. aegyptiaca is preyed on by Novius cardinalis (Mulsant) and N. pumila (Weise) ( Coleoptera : Coccinellidae ) ( Kondo & Watson 2022 in press).
Biology: Monophlebids occur on many host-plant species, mostly woody shrubs and trees. There are 1‒3 generations annually, depending on climatic conditions. Usually, the female has four developmental instars; there is no cyst stage. The male has five instars; unlike in most scale insect families, the male prepupa is quite mobile, with well-developed legs and antennae. In India, D. mangiferae has one generation per year. The eggs, laid in ovisacs in the soil or leaf litter around the host plant, diapause through the winter and hatch early in spring. The crawlers walk up the host plant to the leaves to feed and moult; the sexes are indistinguishable until the third instar. In the male, the prepupa has wing buds and wanders about before forming a waxy test, where it completes development. Adults appear in late spring and mate on the host plant before the females migrate down to the ground to produce ovisacs and eggs ( Karar et al. 2008). Icerya purchasi , which is present in Africa, has a similar life cycle but remains on the plant throughout; the adult “female” (which is a hermaphrodite that can self-fertilise) lays eggs into a fluted wax ovisac attached to both the body and the substrate. Males are rare; they mate with females, but it is not clear whether their sperm are used for reproduction. Inseminated eggs produce hermaphrodites and unfertilised eggs produce males ( Hughes-Schrader 1930, 1963; Normark 2003; Mongue et al. 2021).
Checklist and distributions of Monophlebidae in continental Africa (3 tribes, 13 genera, 71 species, based on García Morales et al. 2016; Gavrilov-Zimin & Stekolshikov 2018, and Watson et al. 2021).
Tribe Drosichini (1 genus, 1 species)
Afrodrosicha nimbae Vayssière : Guinea
Tribe Iceryini (5 genera, 29 species)
“ Crypticerya ” aegyptiensis Foldi: Egypt
“ Crypticerya ” marocensis Foldi: Morocco
“ Crypticerya ” thibaudi Foldi: Egypt
“ Crypticerya ” sp. ( sensu Foldi 2010); undescribed, on Rosa ): Kenya
Gigantococcus alboluteus (Cockerell) : Nigeria
Gigantococcus bicolor (Newstead) : Ghana
Gigantococcus bimaculatus (De Lotto) : Kenya
Gigantococcus brachystegiae (Hall) : Zimbabwe
Gigantococcus cajani (Newstead) : Nigeria
Gigantococcus caudatus (Newstead) : Sierra Leone, Uganda
Gigantococcus euphorbiae (Brain) : Benin, Mozambique, South Africa, Zimbabwe
Gigantococcus ewarti (Newstead) : Nigeria
Gigantococcus gowdeyi (Newstead) : Ghana, Uganda
Gigantococcus longisetosus (Newstead) : Democratic Republic of the Congo, Kenya, Sierra Leone, Tanzania
Gigantococcus maximus (Newstead) : Democratic Republic of the Congo, Gabon, Ghana, Ivory Coast, Kenya, Nigeria, Uganda
Gigantococcus nigroareolatus (Newstead) ; Benin, Democratic Republic of the Congo, Ghana, Tanzania, Uganda
Gigantococcus pattersoni (Newstead) : Ghana, Kenya
Gigantococcus rodriguezi (Castel-Branco) : Mozambique
Gigantococcus schoutedeni (Vayssière) : Democratic Republic of the Congo, Kenya, Senegal
Gigantococcus splendidus (Lindinger) : Tanzania
Gigantococcus sulfureus (Linidinger) : Tanzania, Uganda
Gigantococcus theobromae (Newstead) : Nigeria
Gueriniella serratulae (Fabricius) : Algeria
Icerya aegyptiaca (Douglas) : Egypt, Kenya, Tanzania ( Zanzibar)
Icerya corticalis Vayssière : Democratic Republic of the Congo
Icerya natalensis (Douglas) : South Africa
Icerya purchasi Maskell : Algeria, Angola, Democratic Republic of the Congo, Egypt, Eritrea, Ethiopia, Kenya, Libya, Malawi, Morocco, Mozambique, Senegal, Somalia, South Africa, Sudan, Tanzania (including Zanzibar), Togo, Tunisia, Uganda, Zambia, Zimbabwe
Icerya seychellarum (Westwood) : Egypt, Ethiopia, Kenya, Malawi, South Africa, Uganda
“ Steatococcus ” hystrix Gavrilov-Zimin & Stekolshikov: Mali
Tribe Monophlebini (7 genera, 41 species)
Aspidoproctus armatus Newstead : Democratic Republic of the Congo, Mozambique, Tanzania, Zimbabwe
Aspidoproctus bifurcatus Thorpe : Tanzania, Zimbabwe
Aspidoproctus bouvieri Vayssière : Gabon
Aspidoproctus carinatus (Lindinger) : Tanzania
Aspidoproctus congolensis Vayssière : Democratic Republic of the Congo
Aspidoproctus ellenbergeri Vayssière : Zambia
Aspidoproctus ghesquierei Vayssière : Democratic Republic of the Congo
Aspidoproctus giganteus Newstead : Nigeria
Aspidoproctus glaber (Lindinger) ; Malawi, Zimbabwe
Aspidoproctus magnicornis Thorpe : Uganda
Aspidoproctus maximus (Lounsbury) : Tanzania, Zimbabwe
Aspidoproctus mimeuri Vayssière : Senegal
Aspidoproctus mirabilis (Cockerell) : South Africa, Tanzania
Aspidoproctus neavei Newstead : Malawi
Aspidoproctus pallidus (Newstead) : Tanzania
Aspidoproctus parvus (Lindinger) : Tanzania
Aspidoproctus pertinax (Newstead) : Malawi, Zimbabwe
Aspidoproctus tricornis (Newstead) : Kenya, Malawi, South Africa, Zimbabwe
Aspidoproctus verrucosus Newstead ; Uganda
Aspidoproctus vuileti (Vayssière) : Niger
Aspidoproctus zimmermanni (Newstead) , comb. n. (see below): Tanzania
Monophleboides africanus (Newstead) : Namibia
Monophleboides arachidis Vayssière : Democratic Republic of the Congo
Monophleboides gymnocarpi (Hall) : Egypt
Monophleboides hirtus (Brain) : Malawi, South Africa
Monophleboides sjostedti (Newstead) : Tanzania
Monophleboides suaedae halocnemae (Vayssière) : Tunisia
Monophleboides suaedae suaedae (Vayssière) : Tunisia
Monophlebus dumonti Vayssière : Tunisia
Monophlebus hoggarensis Vayssière ; Algeria
Monophlebus raddoni Westwood : Ghana, Uganda
Palaeococcus fuscipennis (Burmeister) : Algeria
Pseudaspidoproctus acaciae (Joubert) : South Africa
Pseudaspidoproctus africanus (Newstead) : Tanzania
Pseudaspidoproctus fortis (Cockerell) : South Africa
Pseudaspidoproctus fulleri (Cockerell) : Kenya, South Africa
Pseudaspidoproctus hyphaeniacus (Hall) : Egypt, Libya
Pseudaspidoproctus vayssieriellus Ghesquière : Democratic Republic of the Congo, Nigeria
Vrydagha lepesmei Vayssière ; Democratic Republic of the Congo
Walkeriana andreae Green : Democratic Republic of the Congo
Walkeriana digitifrons Newstead : Uganda
Key to most of the known genera of Monophlebidae in continental Africa
1(0) Abdominal spiracles numbering 2‒4 pairs (on posterior abdominal segments). Cicatrices numbering 1‒13. Body covered with hairs and flagellate setae................................................................. tribe Iceryini ... 2
- Abdominal spiracles usually difficult to see, numbering 5‒7 pairs. Cicatrices numbering 1 or 3 or very numerous. Body covered with spines and/or hairs................................................................................ 6
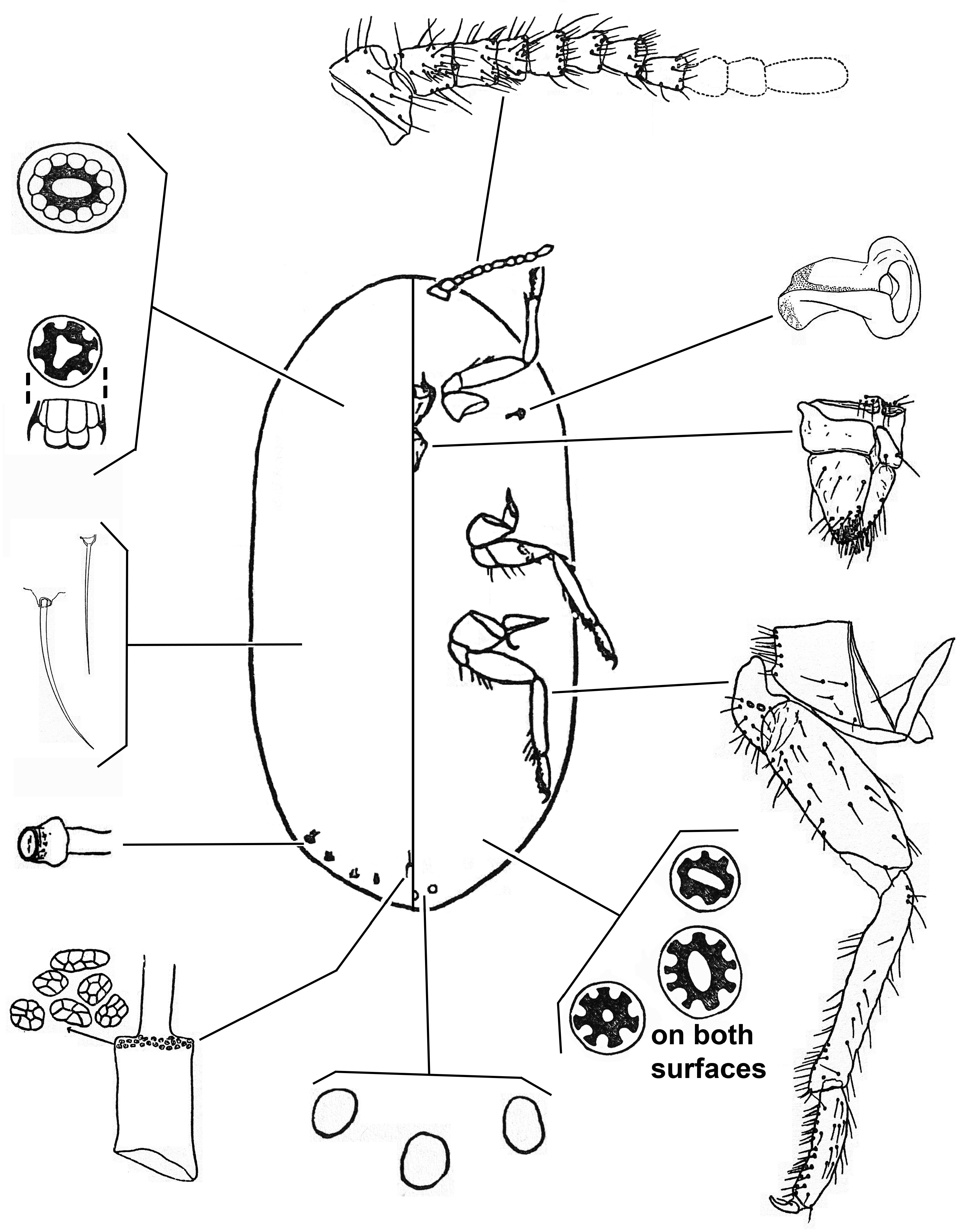
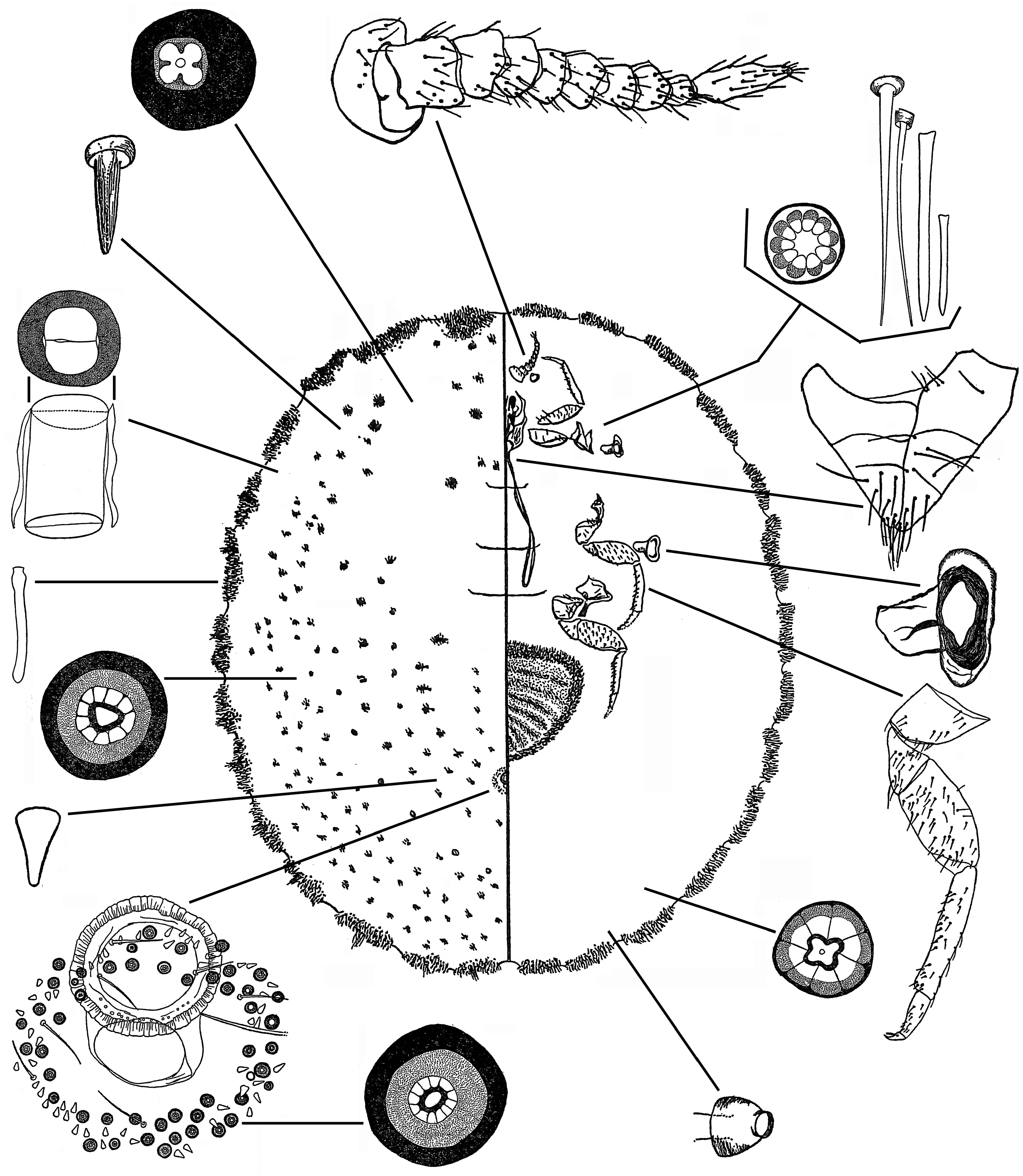
2(1) Abdominal spiracles numbering 4 pairs. Labium long, with 3 segments, the basal segment very narrow. Anal tube inner ring with 2 rows of pores.................................................................. Gueriniella View in CoL ( Fig. 11 View FIGURE 11 )
- Abdominal spiracles numbering 2 or 3 pairs. Labium short and conical, with 2 indistinct segments, the basal segment wide. Anal tube inner ring without pores....................................................................... 3
3(2) Ventral cicatrices numbering 6‒23 posterior to vulva, grouped in a circular area on midline........ “ Crypticerya View in CoL ” ( Fig. 12 View FIGURE 12 )
- Ventral cicatrices numbering 1 or 3 posterior to vulva in a single row across midline................................ 4
4(3) Compound multilocular pores absent. Open-centre pores present or absent........................... Icerya View in CoL ( Fig. 10 View FIGURE 10 )
- Compound multilocular pores present. Open-centre pores absent............................................... 5
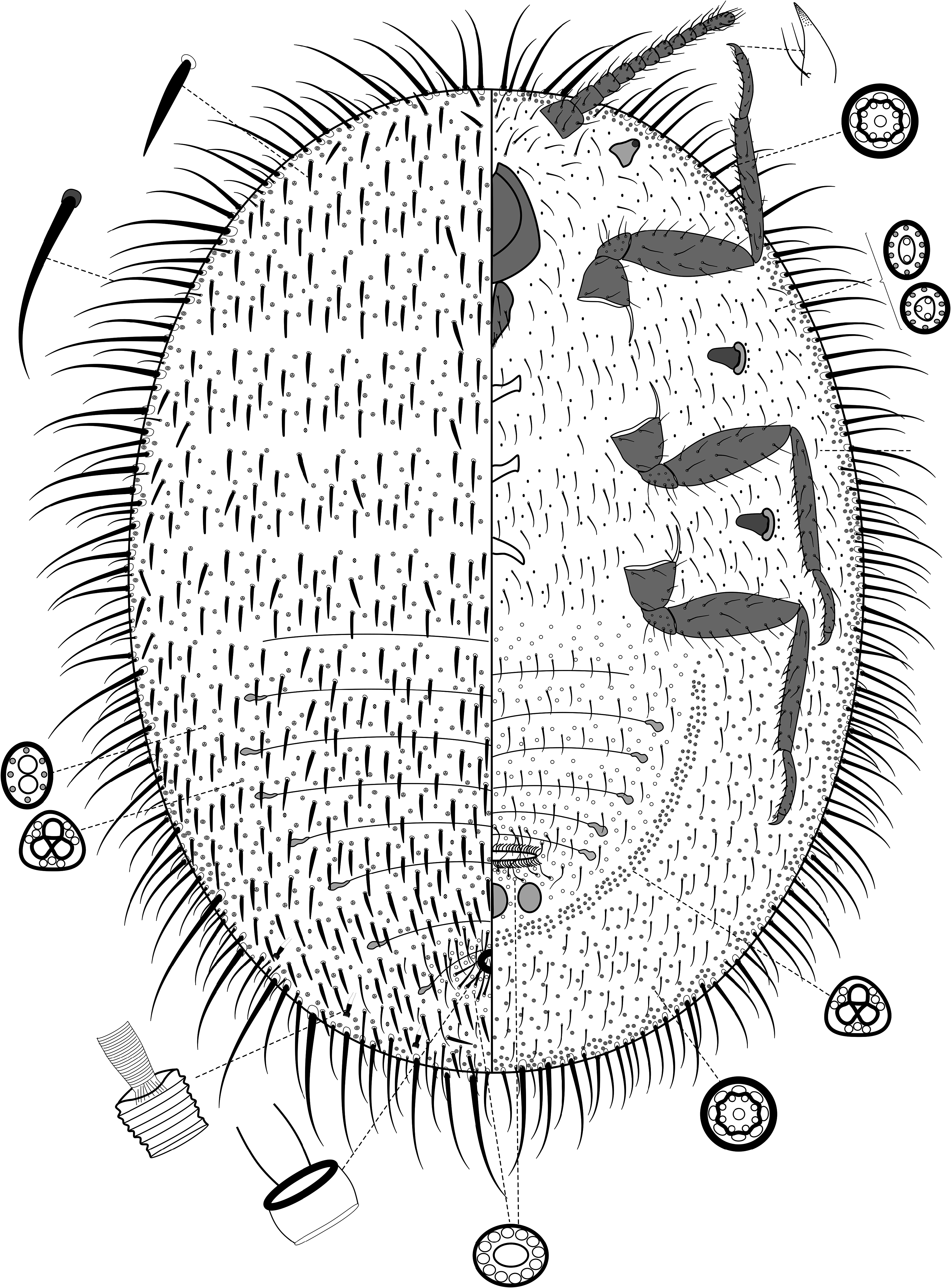
5(4) Dorsal setae robust, spiniform and slightly lanceolate, quite crowded; most setae in marginal areas robust, some up to 5x as long as a dorsal seta, crowded. Marsupium present. With 3 cicatrices....................... “ Steatococcus View in CoL ” hystrix ( Fig. 13 View FIGURE 13 )
- Dorsal setae fine and hair-like, not crowded; setae in marginal areas hairlike, sometimes robust and very long but not crowded. Marsupium present or absent. With 1 or 3 cicatrices...................................... Gigantococcus View in CoL ( Fig. 14 View FIGURE 14 )
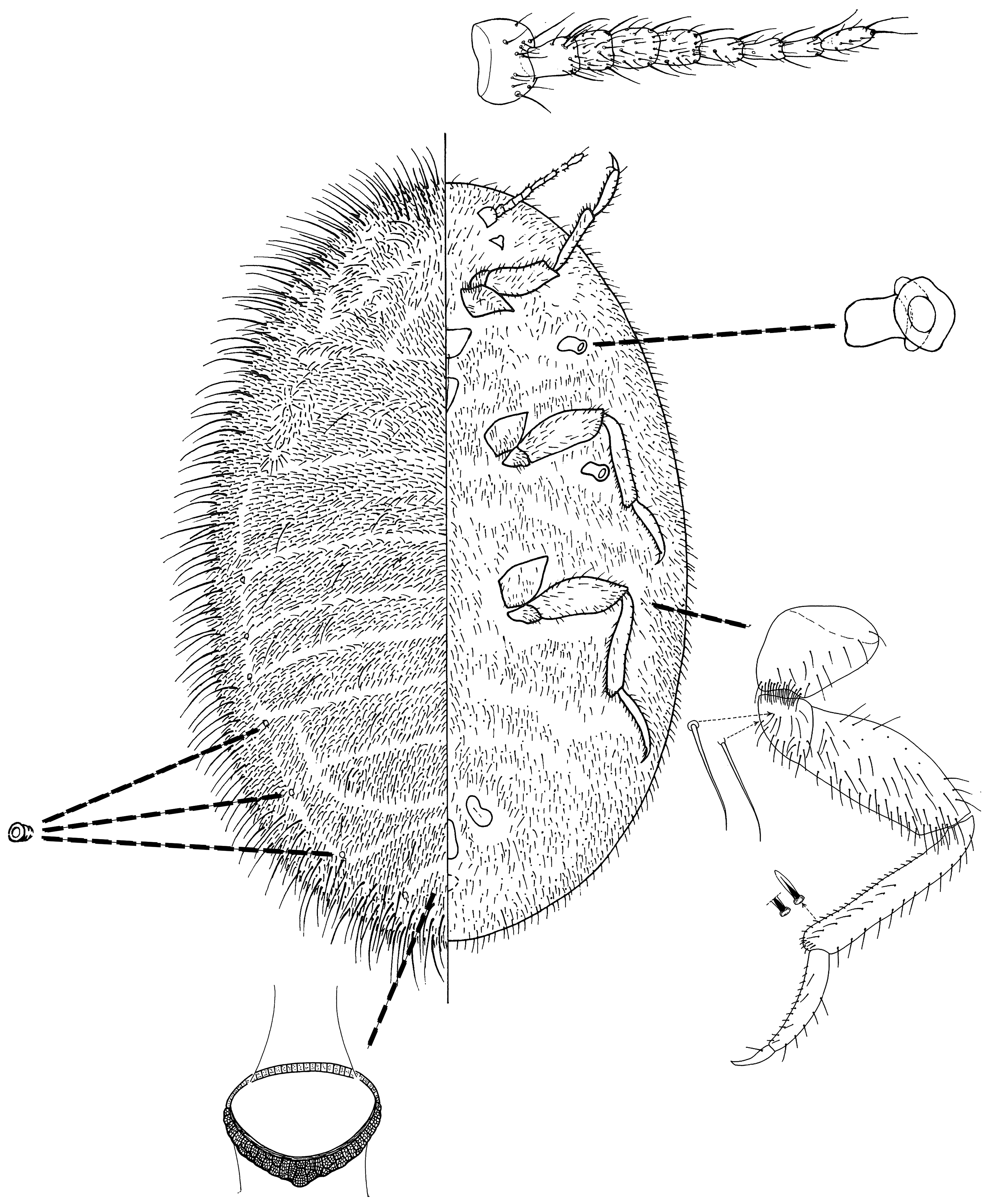
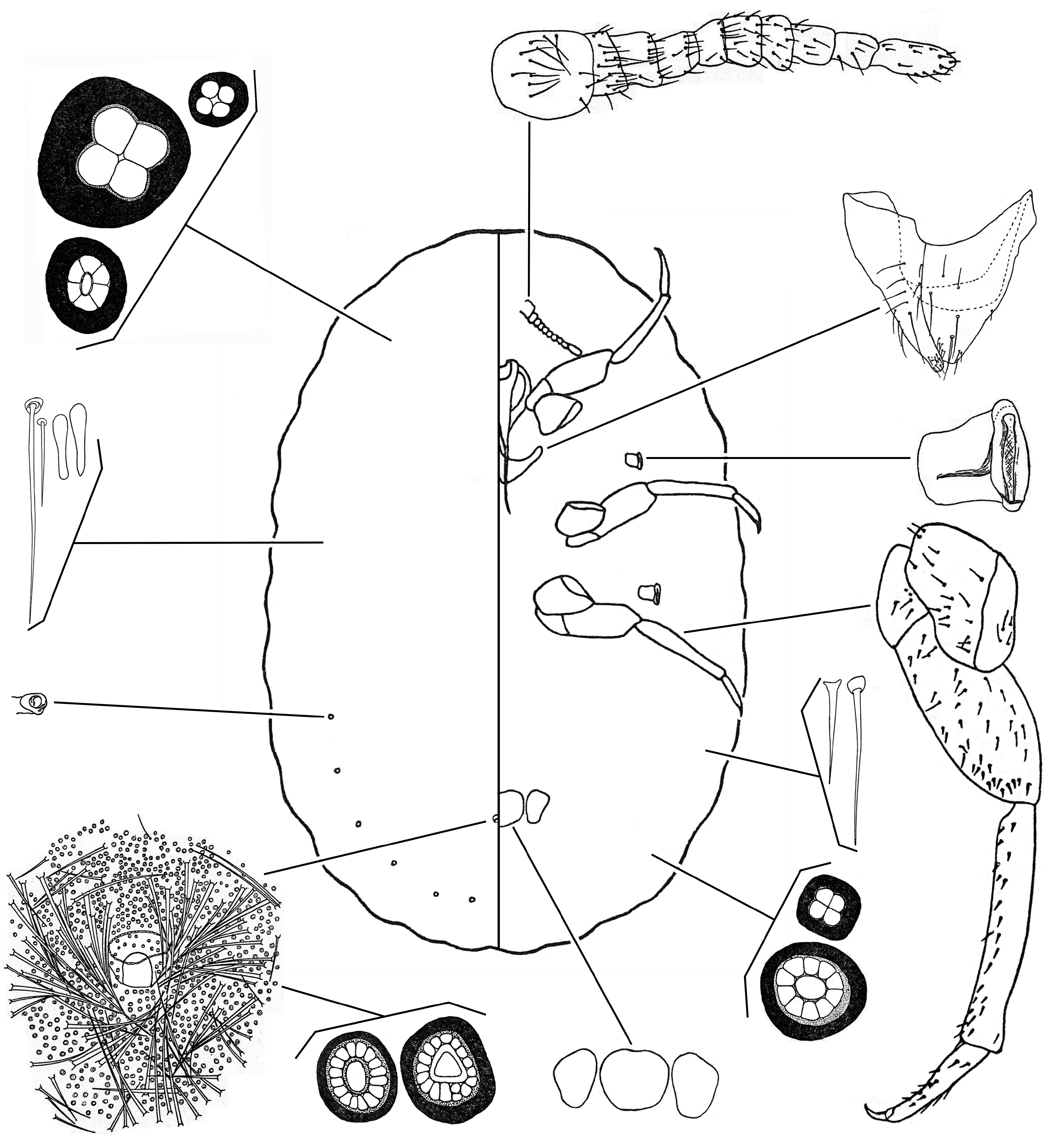
6(1) Body very densely covered with hairs, all same type, finely pointed. Antenna usually with 8 or 9 segments.................................................................................. tribe Drosichini ... Afrodrosicha View in CoL ( Fig. 15 View FIGURE 15 )
- Body with hairs and/or spines, often of more than 1 type, not crowded over entire surface. Antenna with 8‒11 segments..................................................................................... tribe Monophlebin ... 7
7(6) Claw with 3 denticles. Multilocular pores of 2 types, some with large loculi, some with small loculi. Palaeococcus View in CoL ( Fig. 16 View FIGURE 16 )
- Claw without denticles. Multilocular pores of 1 or more types................................................. 8
8(7) Ventral cicatrices very numerous on head, thorax and abdomen, forming irregular rows that form a broad submarginal band on each side. Ventral marsupial opening relatively small and subcircular, bordered by a dense band of disc pores. In life, marsupial opening normally covered by a white layer of felted wax................................... Aspidoproctus View in CoL ( Fig. 17 View FIGURE 17 )
- Ventral cicatrices numbering 1 or 3, situated posterior to vulva in a row across midline. Marsupial opening, if present, often U- or V-shaped and bordered by relatively few pores; not covered with felted wax in life............................ 9
9(8) Body margin with about 12 segmental tubercles on each side of thorax and abdomen, each bearing single or small groups of tubular bilocular pores. Body with numerous, conspicuous quadrilocular pores. Dorsum with patches of densely crowded spines.............................................................................. Walkeriana View in CoL ( Fig. 18 View FIGURE 18 )
- Body margin without tubular bilocular pores or segmental tubercles. Quadrilocular pores, if present, small and inconspicuous. Dorsum without patches of crowded spines............................................................... 10
10(9) Body with at least a few cylindrical spines. Marsupium present. Ventral cicatrices numbering 3 Pseudaspidoproctus View in CoL ( Fig. 19 View FIGURE 19 )
- Body without cylindrical spines. Marsupium absent. Ventral cicatrices numbering 1 or 3........................... 11
11(10) Antenna with 10 segments. Ventral cicatrices numbering 3. Anal tube with internal ring bearing pores.... Vrydagha View in CoL ( Fig. 20 View FIGURE 20 )
- Antenna with 11 segments. With 1 or 3 ventral cicatrices. Anal tube without internal ring of pores.................... 11
12(11) Body margin with large tubular bilocular pores......................................... Monophleboides View in CoL ( Fig. 21 View FIGURE 21 )
- Body margin without large tubular bilocular pores................................................. Monophlebus View in CoL
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |