Megophrys (Xenophrys) monticola ( Günther, 1864 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4523.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:96B7B9E3-9F49-4983-A46C-D29CD6B2EE49 |
|
DOI |
https://doi.org/10.5281/zenodo.6490309 |
|
persistent identifier |
https://treatment.plazi.org/id/03D6878A-FFD1-022F-FF73-F894FE16FE39 |
|
treatment provided by |
Plazi |
|
scientific name |
Megophrys (Xenophrys) monticola ( Günther, 1864 ) |
| status |
|
Megophrys (Xenophrys) monticola ( Günther, 1864) View in CoL
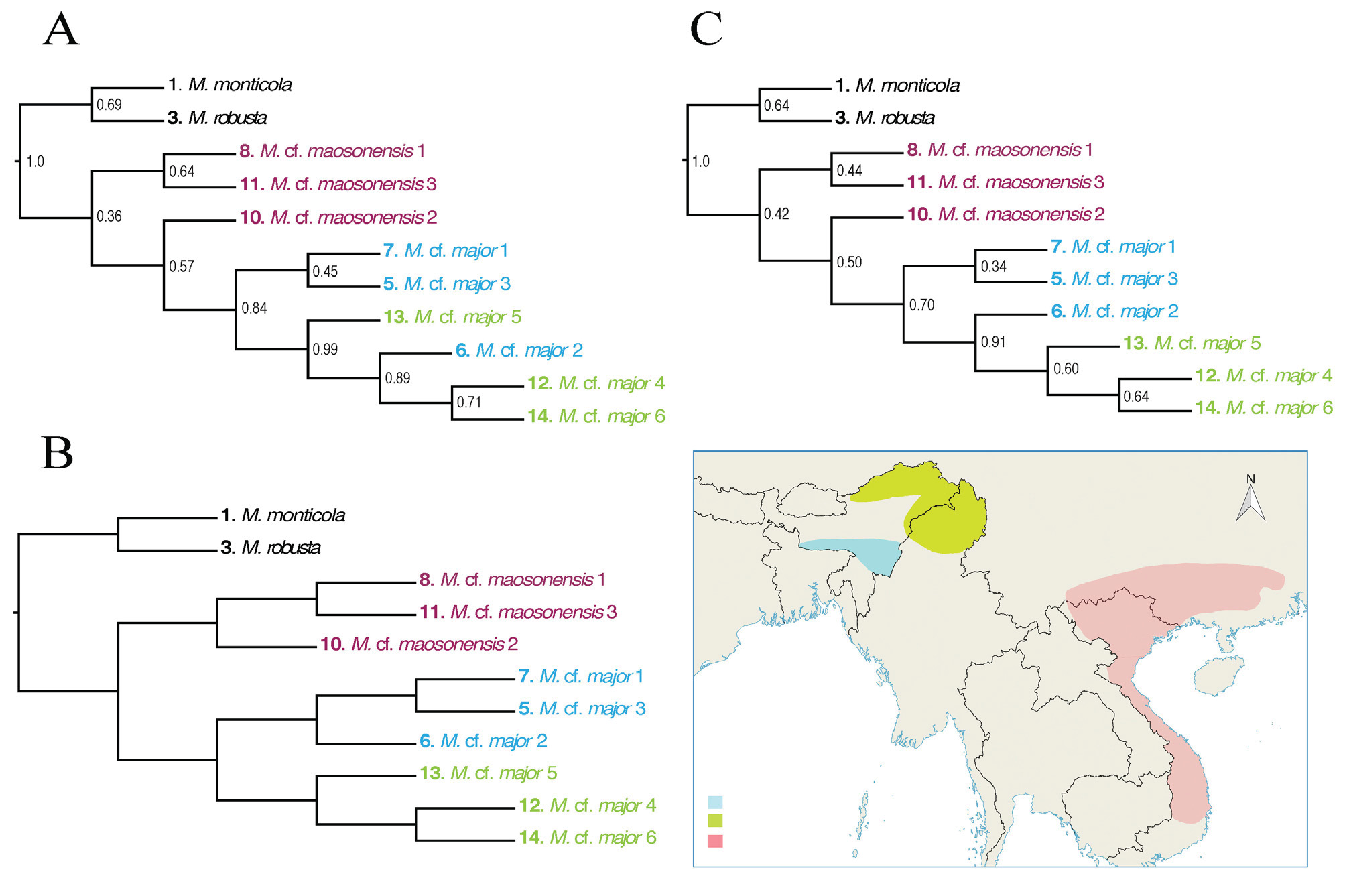
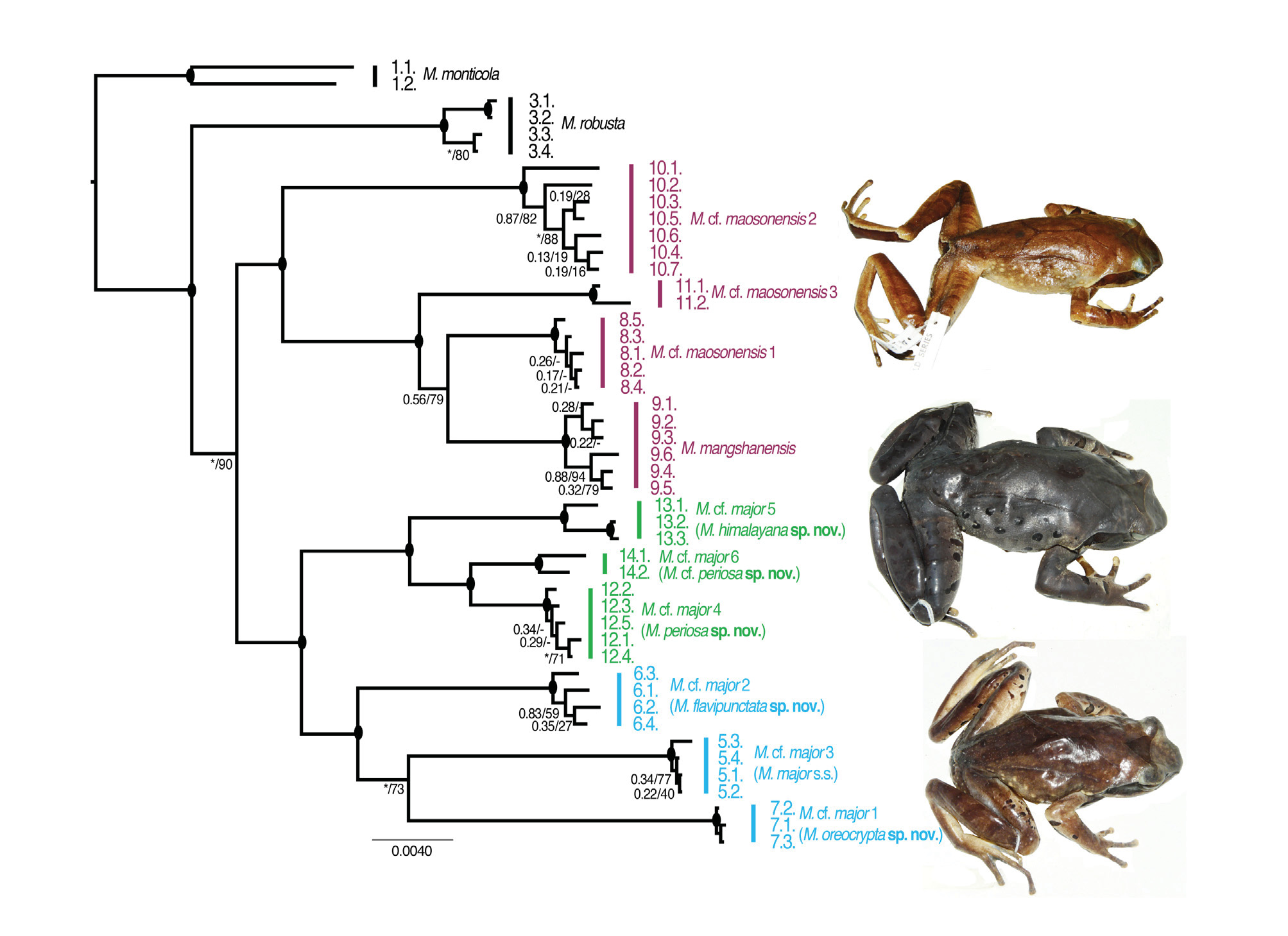
( Figures 6 View FIGURE 6 & 7 View FIGURE 7 ; Table 1)
Xenophrys monticola View in CoL [partim] Günther 1864:415 + pl. xxvi, fig. H, In: The Reptiles of British India. The Ray Society, London: 452 pp. + xxvii + pl. 1–26.
[?] Xenophrys katabhako Deuti et al. 2017:23 , 27–35, In: Nomenclatural puzzle in early Xenophrys nomina ( Anura , Megophryidae ) solved with description of two new species from India (Darjeeling hills and Sikkim). Alytes , 34: 20–48.
[?] Xenophrys sanu Deuti et al. 2017:23 , 27–35, In: Nomenclatural puzzle in early Xenophrys nomina ( Anura , Megophryidae ) solved with description of two new species from India (Darjeeling hills and Sikkim). Alytes , 34: 20–48.
Lectotype of Xenophrys monticola . Adult female (BMNH 1947.2.25.13 [R.R. [18]60.3.19.1336]: Figure 6 View FIGURE 6 ), from “ Sikkim ” state, Northeast India, collected by the Schlagintweit brothers (presumably Hermann), some time between 14 April and 15 August 1855 ( Schlagintweit et al. 1861). Lectotype designation by Mahony et al. (2017). Paralectotype of Xenophrys monticola (non M. monticola s.s.). Adult male (BMNH [18]53.8.12.52), from “Khasya” [Khasi Hills], Meghalaya state, Northeast India, presented by Sir William Jackson Hooker (BMNH Specimen Catalogue), likely collected by his son Sir Joseph Dalton Hooker between June and September 1850 ( Hooker 1854). Paralectotype designation by Mahony et al. (2017).
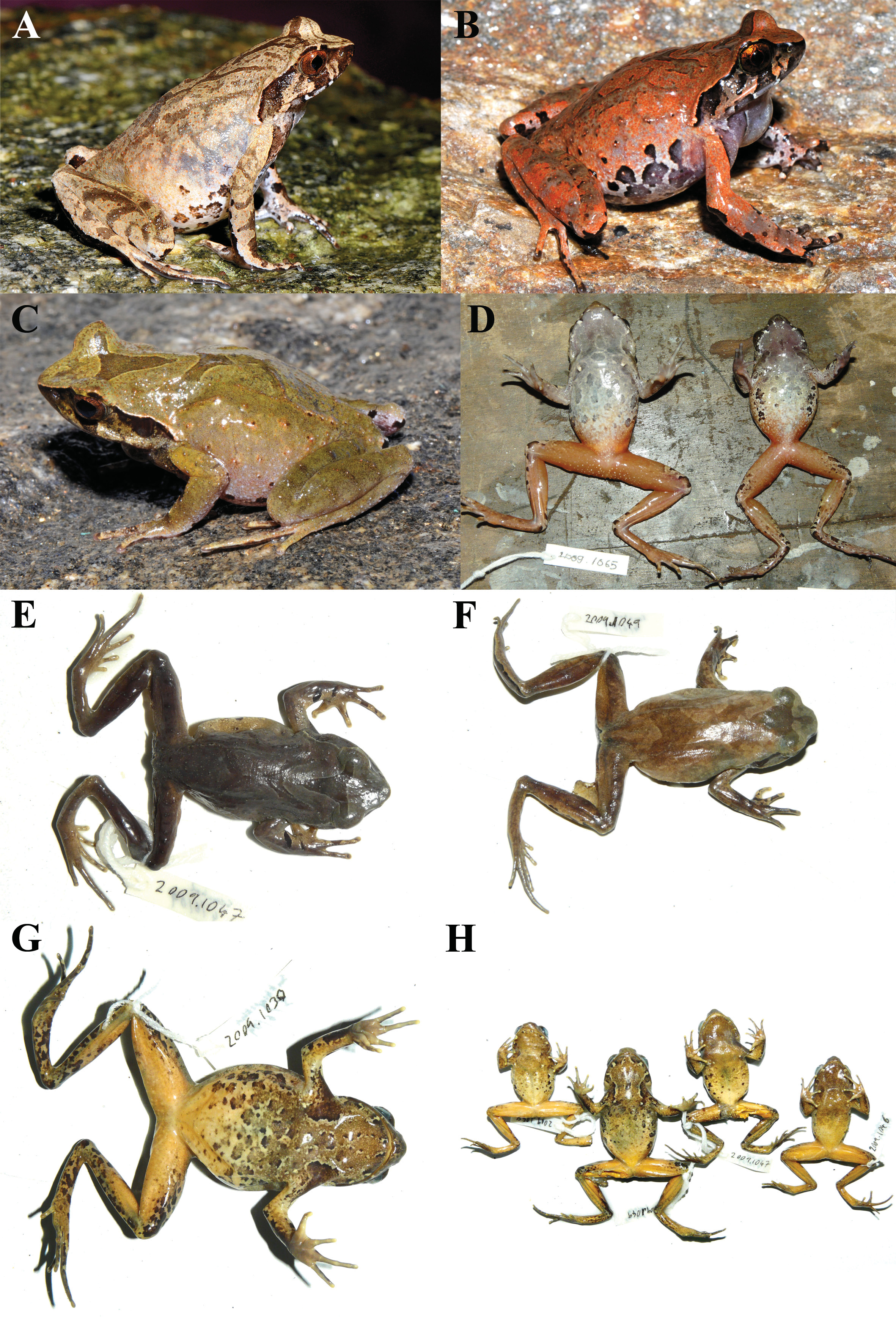
Examined referred specimens. “High-elevation” mitochondrial haplotype (2180–2220 m asl.): three adult males (SDBDU 2011.1046: Figure 7H View FIGURE 7 ; SDBDU 2011.1047: Figure 7C, E & H View FIGURE 7 ; SDBDU 2011.1048: Figure 7H View FIGURE 7 ) and one adult female (SDBDU 2011.1049: Figure 7F & H View FIGURE 7 ), from Senchal Wildlife Sanctuary (26°59'38"N, 88°18'0"E, 2220 m asl.), Rambi (East Range), Darjeeling Sadar sub-division, Darjeeling district, West Bengal state, Northeast India, collected by Systematics Lab members on 0 4 June 2011; three adult males ( SDBDU 2011.429 – 431 ) from Senchal Wildlife Sanctuary (same locality details as above), collected by SDB and RGK on 20 May 2011; one adult male ( SDBDU 2011.1029 ) and one adult female ( SDBDU 2011.1030 : Figure 7A & G View FIGURE 7 ), from Sukhiapokhri-Manebhanjan road (26°59'50.04"N, 88°9'58.56"E, 2180 m asl.), Darjeeling Sadar sub-division, Darjeeling district, West Bengal state, Northeast India, collected by Systematics Lab GoogleMaps members on 0 3 June 2011; one unsexed juvenile ( SDBDU 2011.1418 ), from Bagora Range 8th mile road marker (26°56'44"N, 88°18'00"E, 2180 m asl.), Kurseong sub-division, Darjeeling district, West Bengal state, Northeast India GoogleMaps , collected by SDB and RGK on 20 May 2011. “Mid-elevation” mitochondrial haplotype (880–1135 m asl.): two adult males ( SDBDU 2011.419 & SDBDU 2011.420 ), from Ghoramara area (26°53'19"N, 88°23'53"E, 1110 m asl.), Mahananda Wildlife Sanctuary, Latpanchar, Kurseong sub-division, Darjeeling district, West Bengal state, Northeast India GoogleMaps , collected by SDB and RGK on 19 May 2011; one adult male ( SDBDU 2011.418 ) from Stream 1 (26°54'34"N, 88°23'52"E, 1030 m asl.), Mahananda Wildlife Sanctuary, Latpanchar, Kurseong sub-division, Darjeeling district, West Bengal state, Northeast India GoogleMaps , collected by SDB and RGK on 19 May 2011; three adult males ( SDBDU 2011.1070 – 1072 ) from Mahananda Wildlife Sanctuary (26°53'4.5"N, 88°23'2.46"E, 1080 m asl.), Latpanchar, Kurseong sub-division, Darjeeling district, West Bengal state, Northeast India, collected by Systematics Lab GoogleMaps members on 0 7 June 2011; two adult males ( SDBDU 2011.1056 ; SDBDU 2011.1066 : Figure 7D View FIGURE 7 ) and one adult female ( SDBDU 2011.1065 : Figure 7D View FIGURE 7 ), from Latpanchar town (26°54'34.2"N, 88°23'54.54"E, 1135 m asl.), Kurseong sub-division, Darjeeling district, West Bengal state, Northeast India, collected by Systematics Lab GoogleMaps members on 0 5 and 0 6 June 2011; one adult male ( SDBDU 2011.1423 : Figure 7B View FIGURE 7 ), from Makaibari Tea Estate (26°56'44"N, 88°18'0"E, 880 m asl.), Kurseong sub-division, Darjeeling district, West Bengal state, Northeast India GoogleMaps , collected by SDB and RGK on 21 May 2011. Not assigned to molecular haplotype: adult female lectotype ( BMNH 1947.2.25.13); two adult females ( BMNH [18]72.4.17C; BMNH [18]72.4.17E), from “ Sikkim ” state, Northeast India, collected by T.C. Jerdon , before 1870; one adult male ( BMNH [18]72.4.17G), from “Darjeeling” district, West Bengal state, Northeast India, collected by T.C. Jerdon , before 1870; one adult female ( ZSIC 9650 View Materials ), from “Darjeeling” district, West Bengal state, Northeast India, collected by J. Gammie.
Provisionally referred specimen. One adult male (BMNH [18]72.4.17I), from “Darjeeling” district, West Bengal state, Northeast India, collected by T.C. Jerdon, before 1870.
Lectotype description (measurements in mm). Mature female, SVL 40.5 ( Figure 6 View FIGURE 6 ). General preservation condition poor, dehydrated. Head large, as wide as long (HW 16.2, HL 16.2, IFE 7.2, IBE 11.8); snout rounded in dorsal view, obtuse in lateral view, protruding beyond mandible ( Figure 6C View FIGURE 6 ); rostral appendage absent; loreal region acute and concave with well developed canthus rostralis; dorsal surface of snout concave; eye twice as large as tympanum (EL 5.6, TYD 2.8), slightly longer than snout (SL 5.4); eye–tympanum distance (TYE 2.6) slightly less than tympanum diameter; tympanum oval, obliquely orientated with upper ~20% concealed by supratympanic ridge ( Figure 6C View FIGURE 6 ); pupil indistinct; nostril positioned laterally with raised posterior rim, equidistant from snout tip and anterior edge of eye (EN 2.7, SN 2.8) [presumed artefact of dehydration]; inter-narial distance slightly wider than space between upper eyelids and upper eyelid width (IN 4.9, IUE 4.7, UEW 4.0); pineal ocellus not visible externally; vomerine ridges small, ovoid, acutely angled, positioned equidistant from choanae and each other, situated between to slightly posterior to choanae; vomerine teeth absent; maxillary teeth present; tongue weakly notched posteriorly, medial lingual process absent.
Forelimbs moderately long, thin ( Figure 6A & B View FIGURE 6 ), forearms not enlarged relative to upper forelimbs, slightly shorter than hand length (FAL 9.5, HAL 9.7); fingers long, narrow ( Figure 6D View FIGURE 6 ), finger length formula I<II<IV<III (FIL 3.6, FIIL 4.0, FIIIL 6.8, FIVL 4.2); interdigital webbing, lateral fringes, subarticular and supernumerary tubercles absent; thenar tubercle indistinct, metacarpal tubercles absent; finger tips appear slightly expanded, flattened without pads or terminal grooves. Hindlimbs relatively long, thin ( Figure 6A & B View FIGURE 6 ); thighs shorter than shanks and feet (TL 17.9, SHL 20.4, FOL 19.5); toes long, thin ( Figure 6E View FIGURE 6 ), relative toe lengths I<II<V<III<IV; digit tips appear slightly dilated, flattened, without pads; webbing appears rudimentary; lateral fringes on toes indistinct; outer metatarsal, subarticular and supernumerary tubercles all absent; inner metatarsal tubercle indistinct; ridge of thickened skin absent on ventral surfaces of digits.
Skin texture is not described in detail due to the high level of dehydration ( Figure 6 View FIGURE 6 ), however, generally: dorsal surfaces of body and head (including upper eyelids) appear covered in fine granules; dorsolateral ridges composed of row of fine granules visible on anterior half of body, does not connect with supratympanic ridge on either side; weak V-shaped parietoscapular ridge present, extending posteriorly from temporal region, meeting medially beyond level of forelimbs; supratympanic ridges narrow with minimal widening posteriorly, extends from posterior orbital borders, curves down broadly through upper tympanum, terminating above forelimbs; ventral surfaces and limbs appear primarily smooth; pectoral and femoral glands not distinctly raised, pectoral glands positioned on chest level with axilla, femoral gland positioned on rear of each thigh at midpoint between knee and cloacal opening.
Colouration: In preservative ( Figure 6A & B View FIGURE 6 ): Dorsal surface of head, body and limbs plain brown; faint darker brown triangular marking between eyes; supratympanic ridges bicoloured, upper half white, lower half dark brown; wide slightly oblique dark brown bar on upper lip below orbits ( Figure 6C View FIGURE 6 ); gular region and chest dark brown, with dark and light blotches along edges of gular region; dark brown of chest fades posteriorly on abdomen, with some small dark brown blotches on ventrolateral surfaces of flanks; ventral thighs and shanks primarily light with darker blotches laterally; outer tarsi with continuous dark brown blotch from feet to base of shanks ( Figure 6E View FIGURE 6 ); area surrounding cloaca dark brown; forelimbs brown above with two dark brown transverse stripes on forearms, ventrally light brown; ventral surface of hands and feet faded brown ( Figure 6D & E View FIGURE 6 ); pectoral and femoral glands lighter than surrounding surfaces. In life: Colouration not originally documented.
Description of referred specimen SDBDU 2011.1047 (measurements in mm). Mature male (SVL 40.7) ( Figure 7C, E & H View FIGURE 7 ). Head moderately large, wider than long (HW 15.5, HL 14.9, IFE 7.7, IBE 12.1); snout bluntly pointed in dorsal view, obtusely protruding in lateral view, without rostral appendage ( Figure 7C View FIGURE 7 ); loreal region acute, concave; canthus rostralis angular; dorsal surface of snout concave; eyes more than twice as long as maximum diameter of naked portion of tympanum, and shorter than snout length (EL 5.2, TYD 2.4, SL 5.7); eyetympanum distance (TYE 2.7) slightly longer than maximum diameter of visible portion of tympanum; tympanum oval-shaped and slightly oblique, with upper border concealed by supratympanic ridge ( Figure 7C View FIGURE 7 ); pupils in life oval, horizontally orientated when dilated; nostrils positioned laterally, positioned closer to eyes than to snout tip (EN 2.0, NS 3.3); internarial distance equal to narrowest point between upper eyelids, and greater than eyelid width (IN 4.9, IUE 4.9, UEW 4.3); pineal ocellus not visible externally; vomerine ridges well developed, narrow, weakly raised, orientated acutely, positioned posterior to choanae, closer to choanae than to each other; vomerine teeth small; maxillary teeth present; tongue large, appears rounded posteriorly without notch, medial lingual process absent.
Forelimbs moderately long, thin ( Figure 7C & E View FIGURE 7 ), forearms moderately enlarged relative to upper forelimbs, shorter than hand length (FAL 8.4, HAL 11.4); fingers moderately long, narrow, without lateral fringes, finger length formula I=II<IV<III (FIL 4.6, FIIL 4.6, FIIIL 8.1, FIVL 4.9); interdigital webbing, subarticular and supernumerary tubercles absent; outer metacarpal and thenar tubercles weakly developed; finger tips rounded, not expanded relative to digit widths, with poorly defined circular pads, terminal grooves on pads absent. Hindlimbs moderately long, thin ( Figure 7C & E View FIGURE 7 ); thighs as long as feet, and shorter than shanks (TL 17.9, FOL 17.9, SHL 18.9); toes long, without lateral fringes, relative toe lengths I<II<V=III<IV; toe tips rounded, not dilated, with weakly defined longitudinally oval-shaped pads, terminal grooves on pads absent; inner metatarsal tubercle weak, without distinct borders; webbing, subarticular, supernumerary and outer metatarsal tubercles absent; callous tissue absent on ventral surface of all digits.
Skin of dorsal surfaces of body, upper forelimbs, dorsal and lateral surfaces of head weakly granular ( Figure 7C View FIGURE 7 ); tympanum smooth with borders slightly raised; outer edge of upper eyelids with a short transverse ridge; supratympanic ridges narrow anteriorly gradually expanding posterior to tympanum to become moderately enlarged and glandular, extending obliquely from orbits, abruptly curving above posterior upper border of tympanum, terminating above forelimb insertions; flanks with small to large scattered pustular tubercles; dorsolateral ridges thin, well defined, extending posteriorly from behind supratympanic ridges to ~90% trunk length, non-continuous with gap on anterior third of its length; parietoscapular and sacral ridges weak, not connected, configuration as “> <”; short, obliquely transverse ridges present on dorsal surface of thighs, shanks and forearms; gular region, chest, abdomen and ventral surfaces of limbs smooth; pectoral glands small, weakly raised, level with axilla on chest; femoral glands moderately large, slightly raised, on posterior surface of thighs, subequally distant from knees and cloaca; medium sized white dermal asperities present, forming dense narrow circummarginal band on lower jaw, tympanic region (excluding tympanum), below and including supratympanic ridges and dorsal ridges, sparse on upper eyelids, posterior surfaces of head and anterior dorsum, increasing in density posteriorly to above (but not surrounding regions of) cloaca, few also on tibia, absent from all other surfaces.
Colouration: In preservative ( Figure 7E & H View FIGURE 7 ): Dorsal and lateral surfaces of head, body and limbs primarily dark greyish-brown; light-edged, solid dark brown triangular marking between eyes; distinct dark brown X-shaped marking on dorsum; flank tubercles dark brown with white tips; front of snout and lateral canthus rostralis dark brown; wide vertical dark brown bar below eyes; dark brown blotch extends from posterior canthus through tympanum to posterior supratympanic ridge; two dark brown blotches on anterior lateral surface of forearms; dorsal surface of Finger III with dark brown blotches; lateral surfaces of thighs and shanks with dark brown spots and blotches; throat, chest, anterior abdomen and ventral surfaces of forelimbs, thighs and shanks primarily light brown, suffused with yellow on lateral and posterior abdomen and limbs; some small dark brown spots and blotches on abdomen; area surrounding cloaca and posterior surfaces of thighs dark brown; ventral surfaces of tarsi and feet dark brown with contrasting light grey on toes; hands ventrally mottled yellowish-white and light brown, ventral surfaces of digits light grey; femoral glands creamish-white. In life ( Figure 7C View FIGURE 7 ): Markings as described in preservative, but general dorsal colouration light olive green with dorsal ridges, flank tubercles and larger granules suffused with orange; iris colour golden-brown; throat and chest dark brown fading to white posteriorly on abdomen; groin and inner thighs deep reddish-orange.
Variation. See Table 1 for morphometric variation between the lectotype and referred specimens consisting of 17 adult males, seven adult females, and one juvenile. The remaining referred specimens resemble the lectotype and referred specimen (described above) for most morphological characters, with some exceptions: relative finger length formula varies considerably between I<II<IV<III, I=II<IV<III, I=II=IV<III, IV<I=II<III; occasionally toe III=V in length; vomerine ridge varies from small to medium sized, round to ovoid, positioned between to slightly posterior to level of choanae, occasionally appear to be slightly closer to choanae than to each other; small vomerine teeth present on most specimens; posterior edge of tongue weakly notched on most specimens, but appears rounded on others (likely artefact of preservation); dorsolateral ridge length varies from ~50 to 95% trunk length, can be prominently raised on some individuals; supratympanic ridges on most specimens broadly curve through upper portion of tympanum, concealing up to ~30% of tympanum; following parietoscapular-sacral ridge configurations were observed: “> <”, “> (”, “>–<”, “>–|”, “>- <”; dermal asperities coverage on males similar to that on referred specimen, typically varying in density of coverage on some surfaces, however asperities also found sparsely covering dorsal surfaces of thighs, tarsi, forelimbs, and on surrounding regions of cloaca; females have considerably less dermal asperities, juveniles have none; outer metacarpal and thenar tubercles moderately well developed on some individuals, weak inner metacarpal tubercle visible on some specimens; finger and toe tips very slightly expanded on some specimens. Distinct X-shaped dorsal marking present on bodies of approximately half of specimens; dorsal colouration varies between light yellowish-brown, orange-brown ( Figure 7B View FIGURE 7 ), greyish-brown, or olive green ( Figure 7C View FIGURE 7 ); groin and ventral surface of hindlimbs on many males and one female light to deep reddish-orange in life ( Figure 7D View FIGURE 7 ); ventral markings vary considerably with small to large, dense to sparse blotching on abdomen ( Figure 7D, G & H View FIGURE 7 ), ventral surfaces of hindlimbs may be plain (without markings) or densely mottled with dark brown blotches, throat may be pale brown with distinct large darker blotches, or almost plain dark brown.
Secondary sexual characters. Males: weak to moderately raised nuptial pads present, covered with black micro-asperities, covering most of dorsal surface of Finger I, narrowing distally, extending to base of distal phalange on inner dorsal side; nuptial pad on Finger II small to medium sized, widest proximally, usually extending to mid-proximal phalange on inner side; large subgular external vocal sac distinct as loose skin on some specimens; internal vocal slits present near rear of mandible; forearms slightly to moderately enlarged relative to upper forelimbs. Females: mature ova without pigmented poles (diameter <3 mm); nuptial pads, vocal sac, internal vocal slits, enlarged forearms, all absent.
Morphological comparison. Megophrys monticola (adult males N =17 [excluding the provisionally referred specimen], adult females N =7) differs from M. mangshanensis by absence of distinct white upper lip stripe (vs. present). For comparisons with subsequent species covered in this paper, refer to relevant morphological comparison sections for those species.
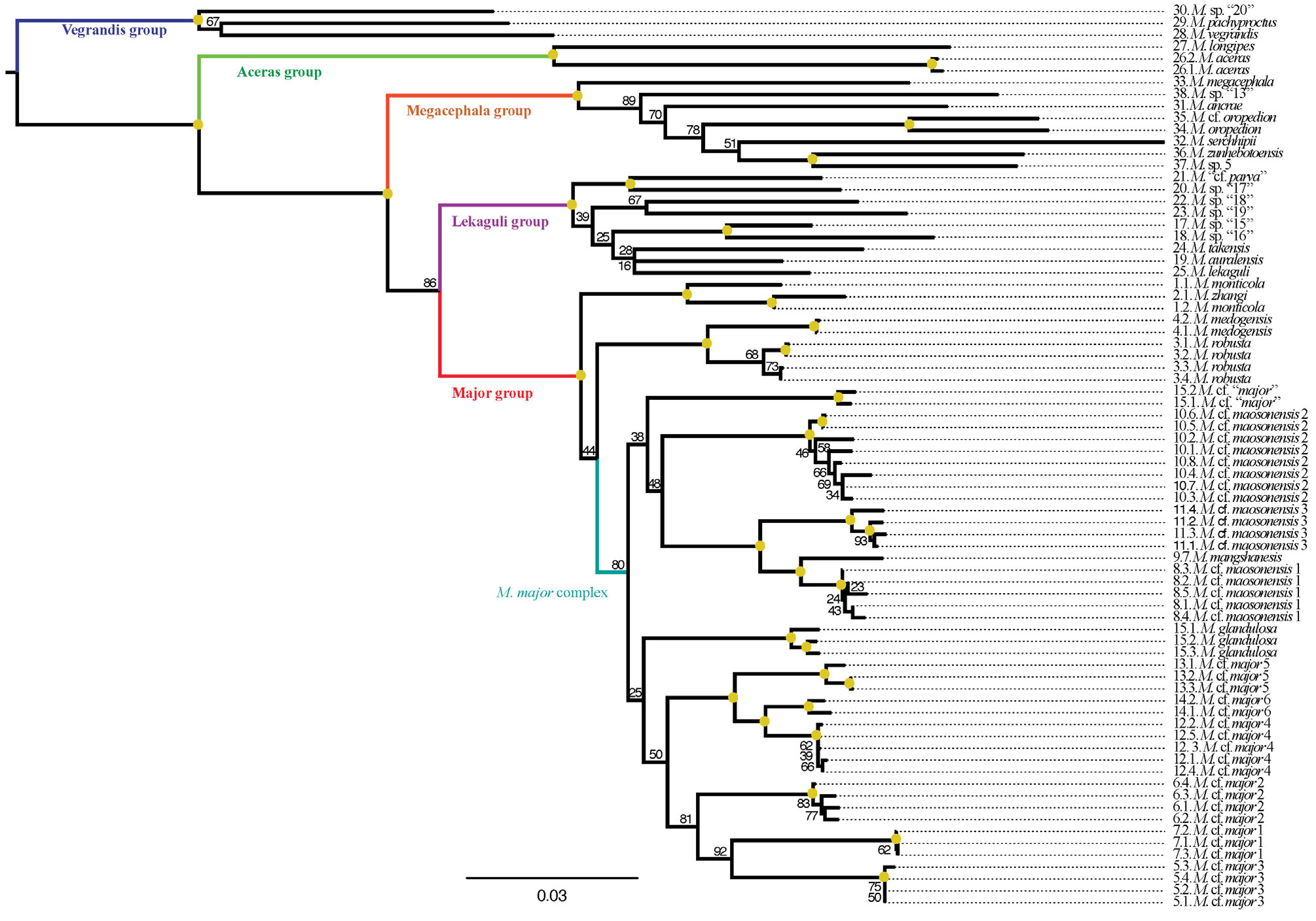
Systematic position. Megophrys monticola was found to be the sister taxon to all remaining species in the MMSG ( Mahony et al. 2017; Figures 2 View FIGURE 2 , 3 View FIGURE 3 & 4 View FIGURE 4 ). In Darjeeling, this species has two comparatively deeply divergent mitochondrial haplotypes (Appendix I, Table 6; Appendix II, Figures 1 View FIGURE 1 , 5 View FIGURE 5 , & 7). However, nuclear diversity observed between these populations consisted of pSNPs only ( Figures 3 View FIGURE 3 & 5 View FIGURE 5 ; Appendix II, Figures 3 View FIGURE 3 , 4 View FIGURE 4 , & 6) indicating recent introgression between mid- (880–1135 m asl.) and high-elevation (2180–2220 m asl.) populations. The mid-elevation populations share an identical mitochondrial haplotype with a specimen referred to the species M. zhangi by Chen et al. (2017), collected from the vicinity of its type locality (Appendix II, Figure 5 View FIGURE 5 ). However, the RAG1 sequence of this specimen differs significantly from the Darjeeling populations (figure not provided). Further molecular sampling of intervening populations is necessary to identify the biological processes involved, and the taxonomic significance of the shared mitochondrial DNA between these species. Megophrys monticola (as redefined above) differs from the type specimens of M. zhangi by adult male size (see Morphological comparison section of M. zhangi ), so our current data does not suggest that the distribution of M. zhangi s.s. extends into India.
Etymology. The specific epithet “ monticola ” is a compound Latin word derived from montis meaning “mountains” and - cola meaning “dwelling in”.
Suggested common name: Mountain Horned Frog ( Ahmed et al. 2009).
Distribution. Recent collections of this species were from mid to high-elevation areas (880–2220 m asl.). In the Darjeeling sub-division of Darjeeling district, it was found in Senchal Wildlife Sanctuary, and along the Sukhiapokhri-Manebhanjan road (Rambi range). In the Kurseong sub-division of Darjeeling district, it was found within the Bagora Range, Makaibari Tea Estate, Mahananda Wildlife Sanctuary, and countryside surrounding Latpanchar town. We also include localities provided for Megophrys sanu comb. nov. and Megophrys katabhako comb. nov. which are verifiable by molecular data from Deuti et al. (2017) in the Darjeeling district and neighbouring areas of Sikkim ( Figure 8B View FIGURE 8 ) in anticipation of the conclusions of our taxonomic clarification of these taxa (see Remarks section below). Megophrys monticola appeared to be locally abundant at collection localities and presumably is more widespread in the Darjeeling Hills and neighbouring Sikkim state (e.g., Chanda 1986; Deuti et al. 2017; Subba et al. 2016 [as M. parva ]). This species (as “ parva ”) has been widely reported from Nepal ( Nanhoe & Ouboter 1987; Anders 2002), although at least some of these populations in central and western Nepal may apply to M. zhangi , or similar unnamed species. This species is also likely to range east, at least into Bhutan; a photographed specimen in Wangyal and Gurung (2012) (as X. nankiangensis Liu & Hu, 1966 [ Hu et al. 1966]) appears to be superficially similar to the specimens examined in this study.
Habitat and natural history. Our observations of this species correspond well with details provided by Anders (2002) for the Nepalese populations. Most males in the SDBDU collection were found calling during May and June, along the banks of small temporary and permanent streams (<30 cm to 2 m wide; e.g., Figure 9C View FIGURE 9 ) bordered by dense low vegetation. Here, males typically called from concealed positions on the ground, or from the lower branches of vegetation on the banks. Calling males typically separate themselves from their nearest competitor by at least two meters. Some specimens were collected from roadside streams where the adjacent bank consisted of a near vertical rocky wall, up to 2 m height above stream level, covered with low vegetation and mosses, where males concealed themselves in rocky crags. Females collected in early June contained large ova, indicating that this species breeds at least during the early monsoon season in Northeast India. No tadpoles were observed attributable to this species. Habitats surrounding the collection streams varied from mature primary growth forest to heavily disturbed secondary growth. Refer to Deuti et al. (2017) for further observations.
Remarks. Günther (1864) briefly described Xenophrys monticola as the sole member of the genus Xenophrys Günther, 1864 . The syntype series of X. monticola consisted of two specimens, one from “Sikkim” (a region now encompassing Sikkim state, and Darjeeling and Kalimpong districts of West Bengal state), and the other from “Khasya” (now the Khasi Hills, Meghalaya state). Examination of the two syntypes revealed that they represented two different species ( Mahony et al. 2017). The accompanying figure in Günther’s (1864) original description clearly depicted the Sikkim specimen ( Günther 1864: Pl. XXVI, fig. H; BMNH 1947.2.25.13). In order to provide nomenclatural stability to the genus-level name Xenophrys , restricting the name of the type species to a single biological species was warranted. Mahony et al. (2017) subsequently designated the Sikkim specimen as the lectotype of Xenophrys monticola Günther. The “Khasya” paralectotype (BMNH [18]53.8.12.52) has been provisionally considered to be synonymous with M. parva of eastern Myanmar by Deuti et al. (2017); however, we consider this specimen to be morphologically most similar to the Khasi Hills endemic, Megophrys oropedion Mahony, Teeling and Biju, 2013 .
After its description, Xenophrys monticola was reported from many localities in Northeast India, Nepal, Myanmar, and China (e.g., Boulenger 1882, 1890, 1893, 1899, 1907; Günther 1868; Sclater 1892b). However, its historically recognised extensive distribution is now understood to have resulted from misidentifications of other currently named and unnamed species. Boulenger (1893) described a small Megophrys species from eastern Myanmar as Leptobrachium parvum comparing it with specimens of M. major s.l., which he erroneously considered to represent X. monticola . Boulenger (1908) subsequently placed all megophryids in the genus Megalophrys . He recognised his previous error (noted in his chresonymy of M. major ), and considered X. monticola to be conspecific with M. parva (= L. parvum ), incorrectly retaining M. parva as the valid name for the taxa. Boulenger (1908) might have been led to this nomenclatural decision due to uncertainty regarding the valid name for the species Megophrys montana Kuhl and Van Hasselt, 1822a (also spelled Megalophrys monticola Kuhl & Van Hasselt, 1822b ) that he may have regarded to have been a senior homonym (see Dubois 1982, 1989, 1992 for discussions). Boulenger’s (1908) nomenclatural action went unquestioned and most subsequent reports of X. monticola s.s. in literature were under the name M. parva (e.g., Anders 2002; Boulenger 1908; Bourret 1942; Chanda 1986, 2002; Dutta 1997; Nanhoe & Ouboter 1987; Sarkar et al. 1992; Subba et al. 2016, and many others). Subsequent reports of M. montana Kuhl and Van Hasselt (or M. monticola Kuhl & Van Hasselt ) from Northeast India (e.g., Annandale 1906; Pillai & Chanda 1976, 1979, 1981; Dutta 1997; Sen 2004, and many others) are a result of misidentifications of specimens by authors who have not compared their material with specimens of / literature that provide a morphological description of M. montana s.s. (i.e., no NE Indian Megophrys species possess the large supraocular horn like projections and dorsal ridge configuration of M. montana ), citing papers that have misidentified specimens, or incorrect interpretation of historical taxonomic literature discussing the species. Dubois (1982, 1989) demonstrated that M. monticola Kuhl and Van Hasselt was an incorrect subsequent spelling of M. montana Kuhl and Van Hasselt. Considering Xenophrys monticola and Megophrys parva as conspecifics, Dubois (1992) made a case to the International Commission of Zoological Nomenclature (ICZN) to formally suppress Xenophrys monticola Günther for the purposes of the Principle of Priority (but not for those of the Principle of Homonymy), to retain M. parva due to prevailing usage as the valid name and thus prevent perceived confusion regarding historical literature using the species name “ monticola ”. The ICZN took a formal action on this request in Opinion 1763 ( Anonymous 1994), and added “ Xenophrys monticola Günther ” to the Official Index of Rejected and Invalid Specific Names in Zoology.
Though the type series of M. monticola and M. parva are superficially similar in general appearance, Mahony et al. (2017) indicated that M. parva s.s. is very likely to represent a member of the Southeast Asian endemic M. (Xenophrys) lekaguli species group ( Figure 4 View FIGURE 4 ; Appendix II, Figures 5 View FIGURE 5 & 6 View FIGURE 6 ) based on the molecular systematic position of specimens collected relatively nearby the type locality of M. parva , and that were considered to be morphologically similar to the type specimens. Therefore M. parva s.s. is not conspecific with X. monticola s.s. With this information, it is now obvious that few historical reports of X. “ monticola ” or M. “ parva ” represent M. monticola s.s. as taxonomically redefined here. Moreover, the name M. montana Kuhl and Van Hasselt (/ M. monticola Kuhl & Van Hasselt ) has also been widely used in literature for several other species (e.g., M. ligayae , M. nasuta , M. stejnegeri ) which were recognised as subspecies (after Inger 1954) until relatively recently (e.g., Iskandar 1998). At no point in time, that we are aware of, have both monticola Günther (from Northeast India-Nepal) and the misspelled “ monticola ” Kuhl and Van Hasselt (from Java, Indonesia) been included in the same genus, demonstrating that taxonomic rather than nomenclatural confusion is the primary issue in historical literature. So, we disagree with Dubois (1982, 1989) that widespread confusion from historical literature may be caused by the recognition of M. monticola (Günther) as a valid name. To this effect, an application to revive the combination Xenophrys monticola Günther to represent a distinct species-level taxon will be submitted to the ICZN.
Deuti et al. (2017) described two new species, Megophrys sanu comb. nov. (type locality: “Latpanchar [26°54'34"N, 88°23'53"E, 1110 m], Darjeeling district, West Bengal, India ”) and Megophrys katabhako comb. nov. (type locality: “Kabi [27°41'23"N, 88°62'82"E, 1410 m], North district, Sikkim, India ”). It should be noted that two of three GPS coordinates for tissue sampled specimens provided by Deuti et al. (2017) were found to be erroneous during the preparation of our map ( Figure 8B View FIGURE 8 ), i.e., their longitude coordinates for Kabi were technically not possible since 60 minutes is the maximum in DMS (degrees, minutes, seconds) format: the correct coordinates for Kabi village is ~ 27°24'21"N, 88°37'03"E. Likewise, a stream near Senchal Lake (given as “ 27°06'02"N, 88°17'00"E ”) for ZSIC A 11799 lies nearby Jorethang, Sikkim state, almost 15 km north of the lake (Senchal Lake is at ~ 26°59'37"N, 88°15'54"E). The two new species were recognised based on a morphological examination of recent collections, a comparison with the type specimens of M. monticola and M. parva (amongst others), and DNA barcoding using a short fragment of 16S DNA. Besides those mentioned above, a number of additional errors/oversights in Deuti et al. (2017) are clearly evident, the most obvious of which require discussion here in order to clarify the taxonomic status of their new species. In their molecular analyses, Deuti et al. (2017) included sequences of most of the taxa from the Chen et al. (2017) study except M. zhangi from Tibet. Unusually, M. zhangi appears to also be the only conspecific omitted from the morphological comparisons sections of both Megophrys sanu comb. nov. and M. katabhako comb. nov. ( Deuti et al. 2017). We performed a nucleotide BLAST search of the 16S sequences of Megophrys sanu comb. nov. generated by Deuti et al. (2017) (GenBank numbers KX894678 View Materials –80) and found that they were 99–100% identical to Chen et al. ’s (2017) M. zhangi sequences, and thus are the same as our “mid-elevation” populations associated with M. monticola in this study (refer to the Systematic position section above). We also performed a nucleotide BLAST search of our high-elevation population of M. monticola (GenBank number KY 022312 View Materials from Mahony et al. 2017) and found 98% similarity with the holotype of M. katabhako comb. nov. To further demonstrate the phylogenetic affinities of these two taxa we ran a RAxML analysis that included sequences from Deuti et al. (2017), and additional 12S/12S-tVal-16S sequences we generated for M. monticola populations from the Darjeeling area (Dataset K; Appendix I, Table 2). This analysis demonstrated that M. katabhako comb. nov. and M. sanu comb. nov. are nested within our concept of M. monticola (Appendix II, Figure 7 View FIGURE 7 ).
Measurements of male specimens in this study that genetically corresponded with M. katabhako comb. nov. and M. sanu comb. nov. were not comparable to those provided by Deuti et al. (2017), e.g., the adult male SVL ranges for both species were larger: M. katabhako comb. nov.: 35.0– 37.4 mm N = 3 in Deuti et al. (2017 ––note: despite stating twice in text that four adult males were available, without explanation they provided data for only three in their table 2, Principle Component Analysis, & maybe other statistical analyses) vs. 38.2–42.3 mm N = 7 in our study; M. sanu comb. nov.: 39.0– 46.7 mm N = 5 in Deuti et al. (2017) vs. 42.4–49.5 mm N = 9 in our study. If considered separately, the SVL measurements from our study and Deuti et al. ’s (2017) do correspond with their claim that the two haplotypes differ from each other by adult male body size. In contrast, we found our “high-elevation” haplotype (equivalent to M. katabhako comb. nov.) to have distinctly shorter relative tibia length (average TL/SVL 46.4%, N =7) compared to our “mid-elevation” population (equivalent to M. sanu comb. nov.) (average TL/SVL 49.5% N =9), which is the opposite case reported by Deuti et al. (2017). Keratinised spinules are absent on the ventral surfaces of thighs on all specimens of both “high-” and “mid-elevation” populations in our study, indicating that this character is not diagnostic for M. katabhako comb. nov. We also did not find their stated differences in dorsal colouration and markings to be diagnostic. Further morphometric comparison between the specimens studied in Deuti et al. (2017) and this study is not possible because the authors did not provide measurements for individual specimens, but provided only range, mean and standard deviation for their specimen series.
The morphological differences observed between the series of specimen examined in Deuti et al. (2017) and our study might be the result of sample bias, or differences in measurement techniques/character delineation. However, Deuti et al. (2017) included specimens of two “high-elevation” populations (Ghoom [2448 m] & Kolakham [1860 m]) in their concept of M. sanu comb. nov. without providing supporting molecular data. Their molecular data and ours both associate populations from the vicinity of Ghoom with their M. katabhako comb. nov. so it is possible that their specimen series of M. sanu comb. nov. contains both taxa. Due to the ambiguity of morphologically associating specimens to one or the other of these two mitochondrial haplotypes, and based on our more extensive molecular sampling which suggests elevational segregation of the haplotypes, we recommend that the haplotype association of specimens from localities not sampled molecularly in Deuti et al. (2017) and elsewhere, should be considered unknown.
Deuti et al. (2017), presumably unaware of the lectotypification made by Mahony et al. (2017), lectotypified the “Khasya” syntype (BMNH [18]53.8.12.52) of M. monticola . Since the publication date of Deuti et al. (2017; 31 st July 2017) postdates the publication date of Mahony et al. (2017; 18 th January 2017), the valid lectotype for Xenophrys monticola is that designated by Mahony et al. (2017) (BMNH [18]53.8.12.52 from “ Sikkim ”), according to Article 74.1.1 of the International Code of Zoological Nomenclature, hereafter referred to as the “ Code ” ( ICZN 1999). Without any morphological justification, they assigned the Sikkim syntype (now lectotype) of M. monticola to their referred specimens of M. sanu comb. nov. If their identification was correct, then Megophrys sanu comb. nov. would be considered to represent a junior subjective synonym of Megophrys monticola ( Günther, 1864) .
Deuti et al. (2017) referred to the lectotype of X. monticola as a juvenile female; however, both large and small ova are clearly present in the ovaries, thus we regard this specimen to be an adult. This individual is smaller (SVL 40.5 mm) than adult females of both M. “ katabhako ” comb. nov. (SVL 47.3–48.7 mm, N =2, this study) and M. “ sanu ” comb. nov. (SVL 56.1 mm, N =1, this study; SVL 41.4–60.2 mm, N =5, Deuti et al. 2017:table 2 [note: they list only two adult females in their “Specimens examined” and “Specimens allocated to new species” sections so it is unclear what five ‘adult’ specimens were included in the table]). Based on the available material in our study, the character we found that usually diagnosed the mitochondrial haplotypes is that FIL is less than FIVL on nine of the 10 specimens from our “high-elevation” haplotype (equivalent to M. katabhako comb. nov.) and the M. monticola lectotype. Whereas, on all 10 specimens from our “mid-elevation” haplotype (equivalent to M. sanu comb. nov.) and one specimen of our “high-elevation” haplotype, we found FIVL is less than FIL. We therefore suggest that if the M. monticola lectotype is truly conspecific with either of the two new species described by Deuti et al. (2017), based on morphology alone we consider it to be more similar to the smaller species M. katabhako comb. nov., than to M. sanu comb. nov. We therefore consider Xenophrys katabhako Deuti et al. 2017 to represent a junior subjective synonym of Xenophrys monticola Günther, 1864 .
Our analyses of nuclear markers from one specimen of each of the two mitochondrial haplotypes referring to M. katabhako comb. nov. and M. sanu comb. nov., found no significant divergence between these taxa (see Systematic position section above). An increased mitochondrial and nuclear DNA sampling of both mitochondrial haplotypes from across their ranges will help identify whether our results are an artefact of mitochondrial introgression between two species, e.g., Chen et al. ’s (2017) M. “ zhangi ” and M. monticola s.s., and whether M. monticola as redefined here, represents a single biological species. Therefore, in the absence of evidence suggesting otherwise, and pending further investigations into the taxonomic affinities of populations of small sized Megophrys species that occur west of Bhutan, we recommend that Xenophrys sanu Deuti et al., 2017 and Xenophrys katabhako Deuti et al., 2017 should both be considered as junior subjective synonyms of Megophrys monticola ( Günther, 1864) .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.