Chamaedrilus baekrokdamensis, Dózsa-Farkas & Felföldi & Nagy & Hong, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4496.1.27 |
|
publication LSID |
lsid:zoobank.org:pub:7C536E1E-5D5A-4E2D-9E4F-28F3CEA9664C |
|
DOI |
https://doi.org/10.5281/zenodo.5950205 |
|
persistent identifier |
https://treatment.plazi.org/id/03D3D43A-E444-FFBE-2580-FD4BFDB6FD83 |
|
treatment provided by |
Plazi |
|
scientific name |
Chamaedrilus baekrokdamensis |
| status |
|
Chamaedrilus baekrokdamensis View in CoL sp n.
( Figures 5H View FIGURE 5 , 8–9 View FIGURE 8 View FIGURE 9 )
Chamaedrilus cf. ozenis Torii, 2015 ; Chen et al. 2016, pp. 276-277, Fig. 1A–D View FIGURE 1 .
Type material. Holotype: NIBRIV0000810588, slide No. 2330, adult, stained whole mounted specimen. Type locality: Baekrokdam crater on the summit of Mt. Hallasan, Jeju Island, Korea, soil and litter layers of Abies koreana forest in West slope, N 33˚21'39.2", E 126˚31'51.9", 1862 m asl, 0 9.06.2016, leg. Y. Hong. Paratypes (in total 6 stained, adult specimens on slides and 13 specimens in 70% ethanol): NIBRIV0000810589, slide No. 2286, from type locality, NIBRIV0000811381, slide No. 2190, site 2. P.116.1, slide No. 2289, from type locality, P.116.2.1–P.116.2.3, slide No . 2188–2189, 2191. In 70% ethanol: P.116.3. from type locality 11 specimens; P.116.4 site 11 two specimens.
Further material examined. 7 specimens investigated in vivo, 4 of them processed for DNA analysis.
Etymology. Named after the Baekrokdam crater where it was a very common species (at sites 2 and 3).
Diagnosis. The new species can be recognized by the following combination of characters: (1) about 8–14 mm long 300–350 µm wide in vivo, segments 35–44; (2) chaetae three per bundle, slightly sigmoid without nodulus; (3) clitellum saddle-shaped; (4) three pairs of preclitellar nephridia; (5) coelomocytes elongated oval, transparent; (6) all primary pharyngeal glands free dorsally and without ventral lobes, two compact secondary glands in V and VI; (7) dorsal blood vessel from XIV, blood light pink; (8) sperm funnels cylindrical, 75–110 µm long and 2–3 times longer than wide in vivo, collars wider than funnel body; (9) male copulatory organs compact, well developed, spherical, diameter about 70–100 µm in vivo; (10) spermathecae free, confined to V, ectal ducts distally slightly widening into ampullae and the following tubes widening into thin-walled ental reservoirs in V; at the distal part of ectal ducts near ectal pore with an asymmetrical glandular swelling on its anterior face; (11) one–three large mature eggs at a time.
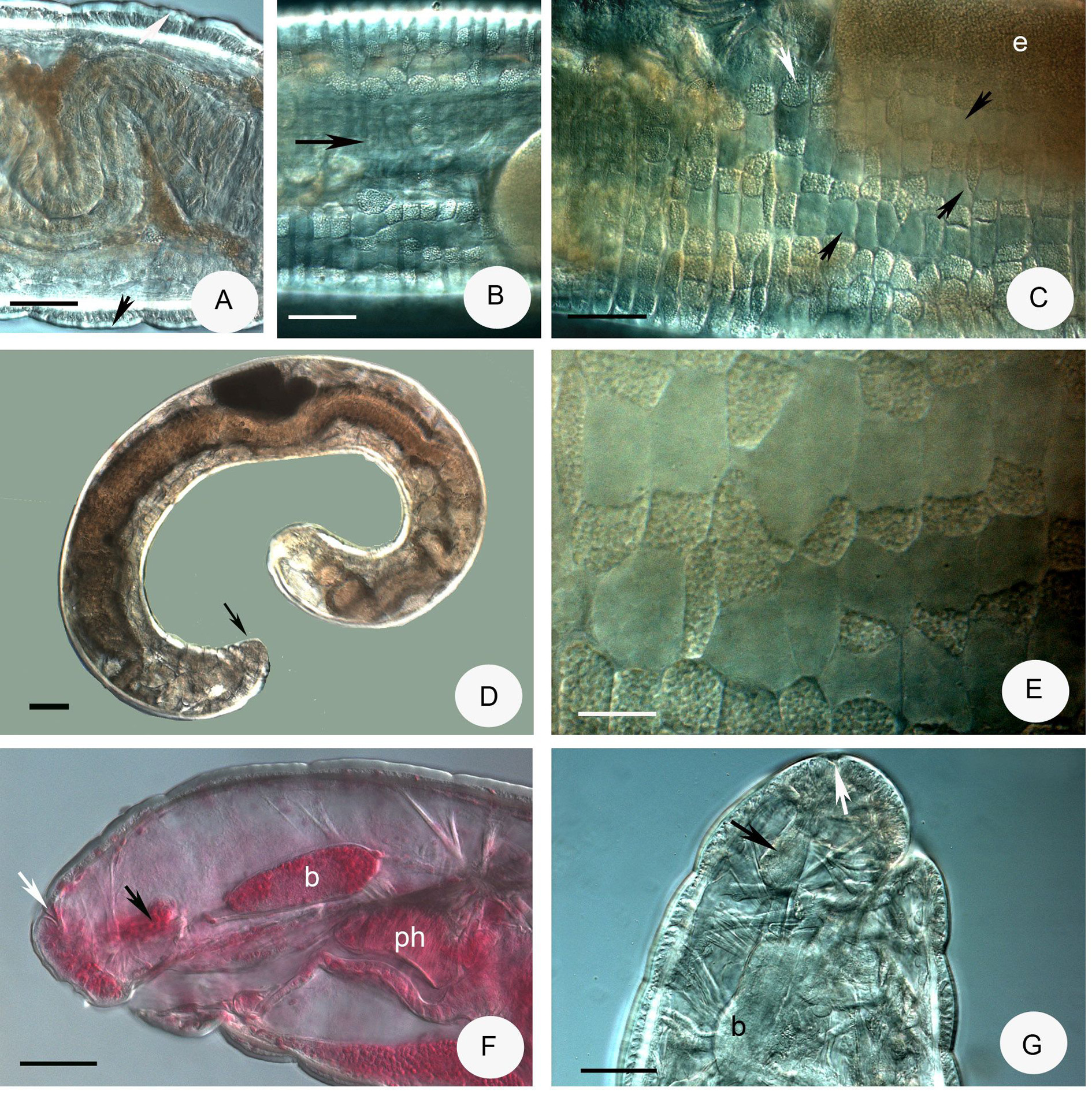
Description. Small worm. Holotype 6.95 mm long, 330 µm wide at VIII and 350 µm at clitellum (fixed), 39 segments. Length of paratypes 8.2–13.5 mm, width 270–350 µm at VIII and 320–430 µm at clitellum, in vivo length of fixed specimens 6–7.8 mm, width 300–350 µm at VIII and 320–350 µm at clitellum, segments (30) 35– 44. Chaetae sigmoid without nodulus. Chaetal formula 3 – 3: 3 – 3, 60 µm in preclitellar region, 75–80 µm in posterior segments three per bundle in all regions, absent in XII. Epidermal glands inconspicuous. Body wall 30– 40 µm thick, cuticle about 1.9–2.5 µm, longitudinal muscle layer well-developed ( Fig. 8C View FIGURE 8 ). Clitellum extending over XII– ½ XIII, saddle-shaped, absent midventrally with about 100 µm distance. Clitellar gland cells irregularly scattered ( Figs. 8A–B View FIGURE 8 ). Head pore 0/I.
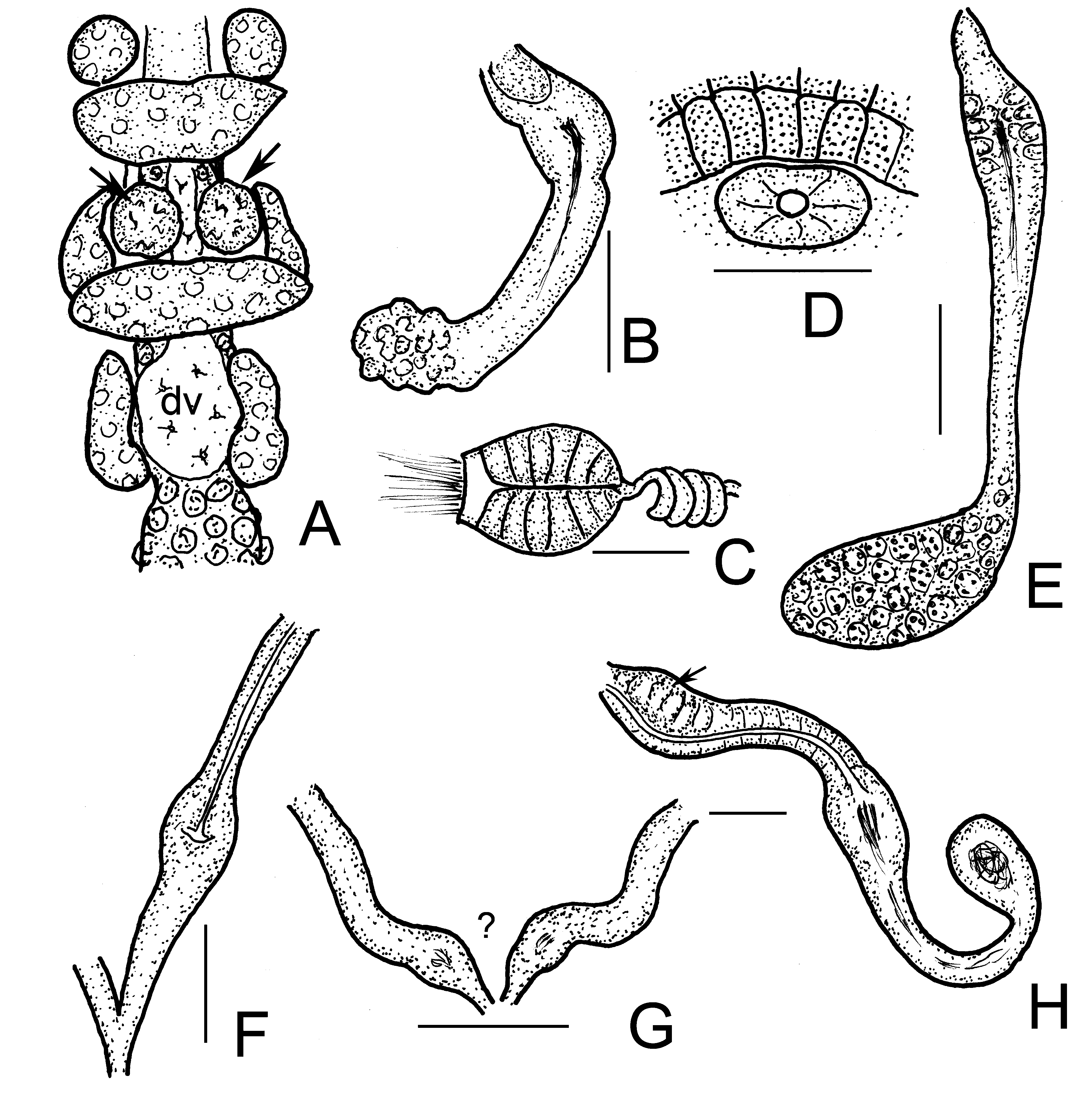
Brain ( Figs. 8D–E View FIGURE 8 ) about 150 µm long and 2 times longer than wide (fixed and in vivo), anteriorly slightly convex, posteriorly incised. Three pairs of primary pharyngeal glands, all free dorsally, without ventral lobes and two pairs of compact secondary glands in V and VI ( Figs. 8H View FIGURE 8 , 9C–D View FIGURE 9 ). Intestine widening in IX. Chloragocytes about 18–28 µm long. Dorsal blood vessel from XIV, blood light pink; the anterior bifurcation in peristomium ( Fig. 8G View FIGURE 8 ). Three pairs of preclitellar nephridia from 6/7 to 8/9; anteseptale consisting of funnel only, efferent duct arises antero-ventrally ( Fig. 8I View FIGURE 8 ). Coelomocytes ( Fig. 8F View FIGURE 8 ) transparent, elongated oval, about 35–40–(55) µm long in vivo (13–15 µm, fixed). Midgut pars tumida not observed. Seminal vesicle small, unpaired in XI, not brown. Sperm funnels ( Figs. 8J View FIGURE 8 , 9A–B View FIGURE 9 ) cylindrical 75–110 µm long and 2–3 times longer than wide in vivo (60–80 µm long and 1.5–2 times longer than wide in fixed specimens; sometimes stubby and about 1.3 times longer than wide only; Fig. 8J View FIGURE 8 ). The collars well-developed 10–18 µm high and wider than the funnel body. Diameter of sperm ducts 6–7 µm. Spermatozoa 70–80 µm long, heads 30–50 µm in vivo. Male glandular bulb compact, well developed spherical, diameter about 70–100 µm in vivo (60–85 µm, fixed). Spermathecae ( Figs. 5H View FIGURE 5 , 9E–F View FIGURE 9 ) confined to V. Ectal ducts thick-walled, 90–100 µm long and 13–18 µm wide in vivo (50–80 µm long and 16–19 µm wide, fixed), distally widening slightly into ampullae (diameter 22–25 µm in vivo), from here and further entally sperm present, arranged in parallel in longitudinal axis of spermatheca. The following tubes 60–80 µm long, slightly narrower, ca. 12–15 µm in vivo (50–80 µm long, 16–18 µm wide, fixed), widening into thin-walled ental reservoirs in V. The reservoirs thin-walled, 35–55 µm long, 1.2–1.5 times longer than wide, with few sperm in lumen. Sometimes the ampulla as wide as the ducts, i.e. not observable. At the distal part of ectal ducts the asymmetrical glandular swelling 47–52 µm long and 35–37 µm high in vivo ( Figs. 5H View FIGURE 5 , 9G View FIGURE 9 ). The canal of ectal ducts 3 µm wide. Near to the opening some smaller glands also observable ( Fig. 9G View FIGURE 9 ). One–three large mature eggs at a time.
Distribution and habitat. In Korea, at site 2, 3 (dominant) and 11, in soil, moss and litter layers under Abies koreana forests and Sorbus alnifolia .
Differential diagnosis. Among the previously described Chaemadrilus species only one species Ch. floridae ( Healy, 1996) has an asymmetrical ectal swelling of the spermathecal ducts as in Ch. baekrokdamensis sp. n. The new species is similar to this species in many other characters, e.g. size, number of chaetae, type of pharyngeal glands, but the principal differences of Ch. floridae to the new species are as follows: sperm funnel 4–5 times longer than wide, and the collar narrower than funnel body vs. only 2–3 times longer than wide and the collar well developed and wider than the funnel body; the coelomocytes are 14–32 µm long, round or oval, finely granular and with prominent nucleus, but larger (35–40–55 µm long in vivo), transparent, elongated oval without conspicuous nucleus in the new species and near to the spermathecal ectal orifice some glands observable, which are present in Ch. baekrokdamensis sp. n. Moreover the first pair of preclitellar nephridia at 6/7 but the number of preclitellar nephridia is not known in Ch. floridae , similarly the first pair at 6/7 and the number of preclitellar nephridia are known (3 pairs) in the new species. Ch. ozenis Torii, 2015 also similar to the new species in more traits (e.g. size, number of segments, number of preclitellar nephridia, the size of sperm funnel), but the most important difference is the absence of asymmetrical glandular swelling at the distal part of ectal ducts. In contradiction, Ch. cf. ozenis described by Chen et al. (2016) is probably identical with Ch. baekrokdamensis sp. n. based on the figures and description, since it has similar size (9–12 mm long 39–42 segments vs. 8.2–13.5 mm, 35–44 in the new species), number of chaetae, identical pharyngeal glands, both have 3 pairs of preclitellar nephridia, the sperm funnel about the same size and 2–3 times longer than wide, and the form of spermathecae is also similar (especially in Fig. 1C View FIGURE 1 of Chen et al. 2016) with the asymmetrical glandular swelling at the distal part of ectal ducts. [It should be noted that in the paper of Chen et al. (2016), in the text Fig. 1 View FIGURE 1 . belongs to Ch. cf. ozenis but according to the legend of Fig. 1 View FIGURE 1 , it is Ch. cf. lapponicus , erroneously].
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.