Scleroderma furfuraceum Rebriev & Zvyagina, 2022
|
publication ID |
https://doi.org/10.11646/phytotaxa.555.2.5 |
|
DOI |
https://doi.org/10.5281/zenodo.6876099 |
|
persistent identifier |
https://treatment.plazi.org/id/03D387C1-FF9E-6614-F9DC-B62E25452246 |
|
treatment provided by |
Plazi (2022-07-21 15:11:17, last updated 2024-11-29 08:32:12) |
|
scientific name |
Scleroderma furfuraceum Rebriev & Zvyagina |
| status |
sp. nov. |
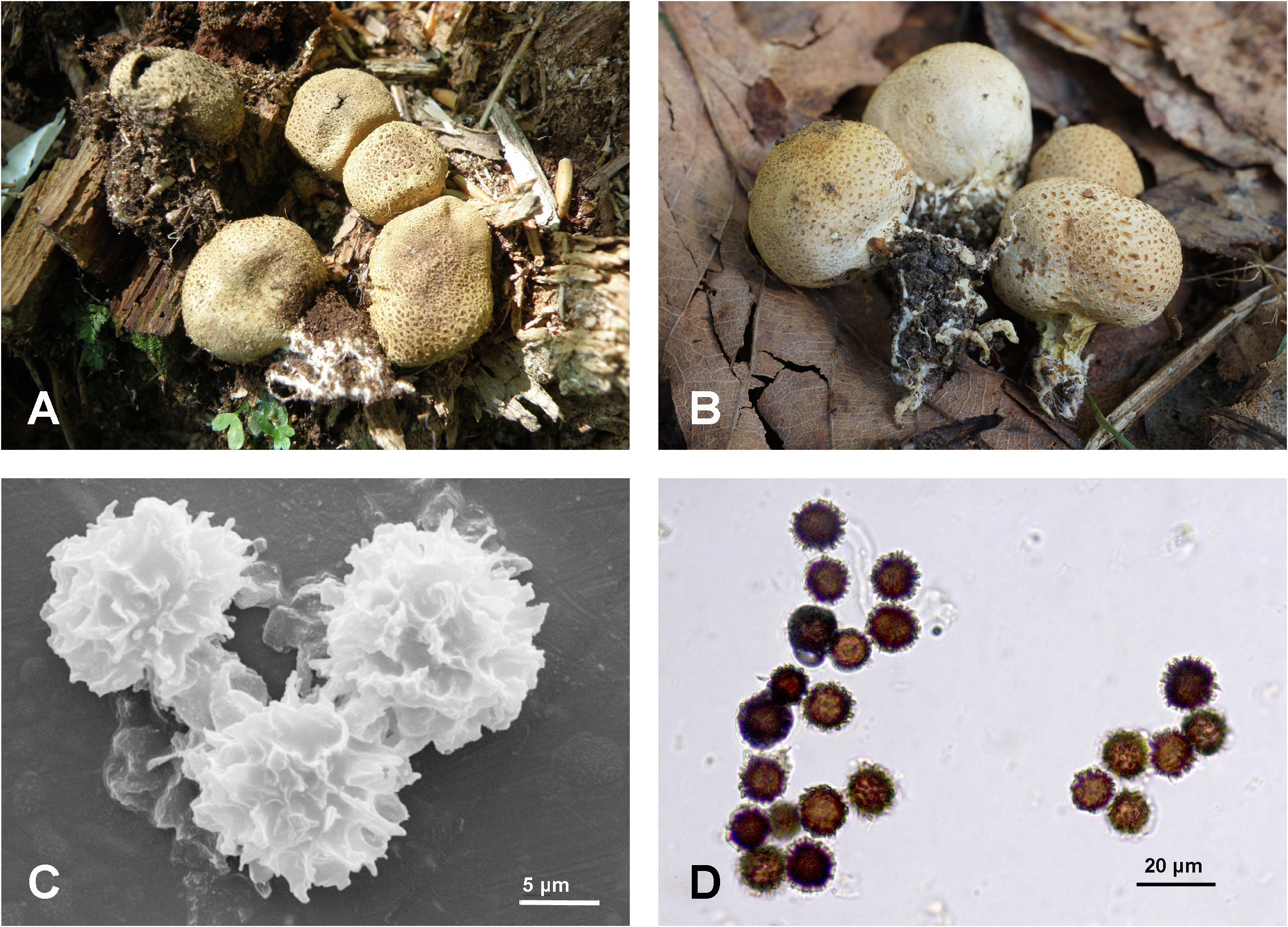
Scleroderma furfuraceum Rebriev & Zvyagina View in CoL , sp. nov. ( Fig. 2 View FIGURE 2 )
Mycobank No: MB 843403.
Diagnosis:— Scleroderma furfuraceum differs from the closest species, S. citrinum , in having smaller basidiomata up to 20 mm diam. and bigger basidiospores (11–) 11.8–16.3 (–17.0) µm diam. (including ornamentation).
Etymology:—‘ furfuraceum ’ refers to the grainy scales of the peridium.
Type:— RUSSIAN FEDERATION. Primorsky Krai, Shkotovsky district, vicinity of Anisimovka village, Litovka mount., camp site Gribanovka , N 43.1302°, E 132.7913°, on well-rotted stump in mixed forest with Quercus mongolica, Pinus koraiensis, Abies holophylla , Tilia amurensis , Betula maximowicziana , Corylus mandshurica , 31 August 2021, Yu. Rebriev (holotype LE-F 342317 ! GoogleMaps , ITS sequence GenBank OM874610 View Materials ).
Description:— Basidiomata small, (12–) 14–17 (–20) mm diam., (9–) 11–13 (–14) mm high, subglobose, with a tuft of mycelial strands, sessile or with a reduced buried pseudostipe. Peridium thin, tough, less than 1– 0.5 mm thick at maturity in the equatorial part of dry specimens and less than 0.1 mm thick near the apical pore, pale yellowish brown (R 230, G 225, B 200 according to Adobe Photoshop CS5 foreground color), yellowish brown (R 248, G 240, B 207) to brown (R 224, G 215, B 158), bearing numerous, small, concolorous to dark reddish brown (R 140, G 90, B 55) scales and warts and consisting of two layers. The outer layer consists of thick-walled yellowish brown to hyaline branched hyphae, on average 3–7 μm diam, with septa and clamp connections. The inner layer consists of thin-walled hyaline branched hyphae on average 5–13 μm diam, sometimes collapsed, with numerous septa and clamp connections. If the scales and warts are worn away a reticulate pattern remains. Dehiscence is by the lacerate irregular apical pore. Gleba solid, dark grey often with violaceous tint when young to pulverulent and grey in maturity, with whitish to creamcoloured plates of trama. Odour not noted.
Basidiospores (11–) 11.8–16.3 (–17.0) μm diam. (including ornamentation), globose, dark brown; surface ornamentation a conspicuous complete to slightly incomplete reticulum, 2.5–3.0 (–4.0) μm high. Basidia not observed.
Ecology: — Solitary or gregarious, on soil, litter or humified wood in broad-leaved and coniferous-deciduous (mixed) forests dominated by Quercus spp . mainly, possibly mycorrhizal with Quercus spp .
Distribution:— S. furfuraceum is commonly distributed in the subtropical Russian Far East as well as in Japan.
Additional specimens examined:— RUSSIAN FEDERATION. Primorsky Krai, Shkotovsky district, vicinity of Anisimovka village, Litovka mount., N 43.1033°, E 132.7829°, on soil in mixed forest with Quercus mongolica, Pinus koraiensis, Abies holophylla , Tilia amurensis , Betula maximowicziana , Corylus mandshurica , 26 August 2021, Yu. Rebriev (LE-F 342313!, ITS sequence GenBank OM874612 View Materials ); Primorsky Krai, Sikhote-Alin Nature Reserve, N 45.2584° E 136.5028°, on soil in mixed forest with Quercus mongolica , Tilia sp. , Acer mono , Pinus sibirica , Abies sibirica , 21 August 2013, O. Morozova (LE-F 342309); ibid., N 44.25849937° E 136.0688°, on soil in mixed forest with Abies nephrolepis , Betula maximowicziana , 05 September 2013, E. Malysheva and V. Malysheva (LE-F 342310!); Primorsky Krai, Chuguevskiy district, Verhneussuriskiy research station, N 44.04° E 134.041°, on soil in Abies - dominated forest, 19 August 1973, E. Bulakh (VLA M 17877!); ibid., on litter in forest with dominance of mixed forest with Quercus mongolica, Pinus koraiensis, 17 September 1974, E. Bulakh (VLA M 17878!); Jewish Autonomous Oblast, Bastak Nature Reserve, up the Bastak river, 49.02°N, 133.03°E, on humified wood in mixed forest, 13 August 2000, E. Bulakh (VLA M 15345!).
Alanbagi, R. A., Alshuwaili, F. E. & Stephenson, S. L. (2019) Fungi associated with forest floor litter in northwest Arkansas. CREAM 9 (1): 25 - 35. https: // doi. org / 10.5943 / cream / 9 / 1 / 3
Baseia, I. G., Silva, B. D. B., Ishikawa, N. K., Soares, J. V. C., Franca, I. F., Ushijima, S., Maekawa, N. & Martin, M. P. (2016) Discovery or Extinction of New Scleroderma species in Amazonia? PLoS ONE 11 (12): e 0167879. https: // doi. org / 10.1371 / journal. pone. 0167879
Brock, P. M., Doring, H. & Bidartondo, M. I. (2009) How to know unknown fungi: the role of a herbarium. New Phytologist 181: 719 - 724. https: // doi. org / 10.1111 / j. 1469 - 8137.2008.02703. x
Crous, P. W., Wingfield, M. J., Richardson, D. M., Le Roux, J. J., Strasberg, D., Edwards, J., Roets, F., Hubka, V., Taylor, P. W. J., Heykoop, M., Martin, M. P., Moreno, G., Sutton, D. A., Wiederhold, N. P., Barnes, C. W., Carlavilla, J. R., Gene, J., Giraldo, A., Guarnaccia, V., Guarro, J., Hernandez-Restrepo, M., Kolarik, M., Manjon, J. L., Pascoe, I. G., Popov, E. S., Sandoval-Denis, M., Woudenberg, J. H. C., Acharya, K., Alexandrova, A. V., Alvarado, P., Barbosa, R. N., Baseia, I. G., Blanchette, R. A., Boekhout, T., Burgess, T. I., Cano-Lira, J. F., Cmokova, A., Dimitrov, R. A., Dyakov, M. Yu., Duenas, M., Dutta, A. K., Esteve-Raventos, F., Fedosova, A. G., Fournier, J., Gamboa, P., Gouliamova, D. E., Grebenc, T., Groenewald, M., Hanse, B., Hardy, G. E. St. J., Held, B. W., Jurjevic, Z., Kaewgrajang, T., Latha, K. P. D., Lombard, L., Luangsa-ard, J. J., Lyskova, P., Mallatova, N., Manimohan, P., Miller, A. N., Mirabolfathy, M., Morozova, O. V., Obodai, M., Oliveira, N. T., Ordonez, M. E., Otto, E. C., Paloi, S., Peterson, S. W., Phosri, C., Roux, J., Salazar, W. A., Sanchez, A., Sarria, G. A., Shin, H. - D., Silva, B. D. B., Silva, G. A., Smith, M. Th., Souza-Motta, C. M., Stchigel, A. M., Stoilova-Disheva, M. M., Sulzbacher, M. A., Telleria, M. T., Toapanta, C., Traba, J. M., Valenzuela-Lopez, N., Watling, R. & Groenewald, J. Z. (2016) Fungal Planet description sheets: 400 - 468. Persoonia 36: 316 - 458. https: // doi. org / 10.3767 / 003158516 X 692185
Csizmar, M., Cseh, P., Dima, B., Orloci, L. & Bratek, Z. (2021) Macrofungi of urban Tilia avenues and gardens in Hungary. Global Ecology and Conservation 28: e 01672. https: // doi. org / 10.1016 / j. gecco. 2021. e 01672
Frank, J. L., Barry, S. & Southworth, D. (2006) Mammal mycophagy and dispersal of mycorrhizal inoculum in Oregon white oak woodlands. Northwest Science 80 (4): 264 - 273.
Haelewaters, D., Dirks, A. C., Kappler, L. A., Mitchell, J. K., Quijada, L., Vandegrift, R., Buyck, B. & Pfister, D. H. (2018) A preliminary checklist of fungi at the Boston Harbor Islands. Northeastern Naturalist 25 (9): 45 - 76. https: // doi. org / 10.1656 / 045.025. s 904
Kumla, J., Suwannarach, N., Bussaban, B. & Lumyong, S. (2013) Scleroderma suthepense, a new ectomycorrhizal fungus from Thailand. Mycotaxon 123: 1 - 7. https: // doi. org / 10.5248 / 123.1
Miyamoto, Y., Terashima, Y. & Nara, K. (2018) Temperature niche position and breadth of ectomycorrhizal fungi: Reduced diversity under warming predicted by a nested community structure. Global Change Biology 24 (12): 5724 - 5737. https: // doi. org / 10.1111 / gcb. 14446
Montagner, D. F., Coelho, G., Silveira, A. O., Baldoni, D. B. & Antoniolli, Z. I. (2015) Morphological and molecular analyses in Scleroderma (Basidiomycota) associated with exotic forests in Pampa biome, southern Brazil. Mycosphere 6 (3): 337 - 344. https: // doi. org / 10.5943 / mycosphere / 6 / 3 / 9
Nouhra, E. R., Hernandez Caffot, M. L., Pastor, N. & Crespo, E. M. (2012) The species of Scleroderma from Argentina, including a new species from the Nothofagus forest. Mycologia 104 (2): 488 - 495. https: // doi. org / 10.3852 / 11 - 082
Palmer, J. M., Lindner, D. L. & Volk, T. J. (2008) Ectomycorrhizal characterization of an American chestnut (Castanea dentata) - dominated community in Western Wisconsin. Mycorrhiza 19 (1): 27 - 36. https: // doi. org / 10.1007 / s 00572 - 008 - 0200 - 7
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M. A. & Huelsenbeck, J. P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61 (3): 539 - 542. https: // doi. org / 10.1093 / sysbio / sys 029
Zhang, Y. Z., Sun, C. Y., Sun, J., Zhang, K. P., Zhang, H. S., Guo, X., Zhou, Y. J., Zheng, D. S. & Li, H. J. (2020) Scleroderma venenatum sp. nov., S. venenatum var. macrosporum var. nov. and S. suthepense new to China. Phytotaxa 438 (2): 107 - 118. https: // doi. org / 10.11646 / phytotaxa. 438.2.4
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
1 (by plazi, 2022-07-21 15:11:17)
2 (by ExternalLinkService, 2022-07-21 15:18:31)
3 (by ExternalLinkService, 2022-07-21 15:28:35)
4 (by ExternalLinkService, 2022-07-21 16:53:40)
5 (by jonas, 2022-07-25 14:14:06)
6 (by ExternalLinkService, 2022-07-25 14:19:07)
7 (by ExternalLinkService, 2022-07-25 14:39:10)
8 (by plazi, 2023-11-07 08:52:54)