Mixophyes australis, Bertozzi & Guzinski, 2023
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5297.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:564FF0B3-96C1-4EEA-9A06-C2C6ABA80B34 |
|
DOI |
https://doi.org/10.5281/zenodo.8006837 |
|
persistent identifier |
https://treatment.plazi.org/id/03D34843-FFD8-2A08-FF2F-9770FF4EFC7E |
|
treatment provided by |
Plazi |
|
scientific name |
Mixophyes australis |
| status |
sp. nov. |
Mixophyes australis sp. nov.
Southern Stuttering Frog
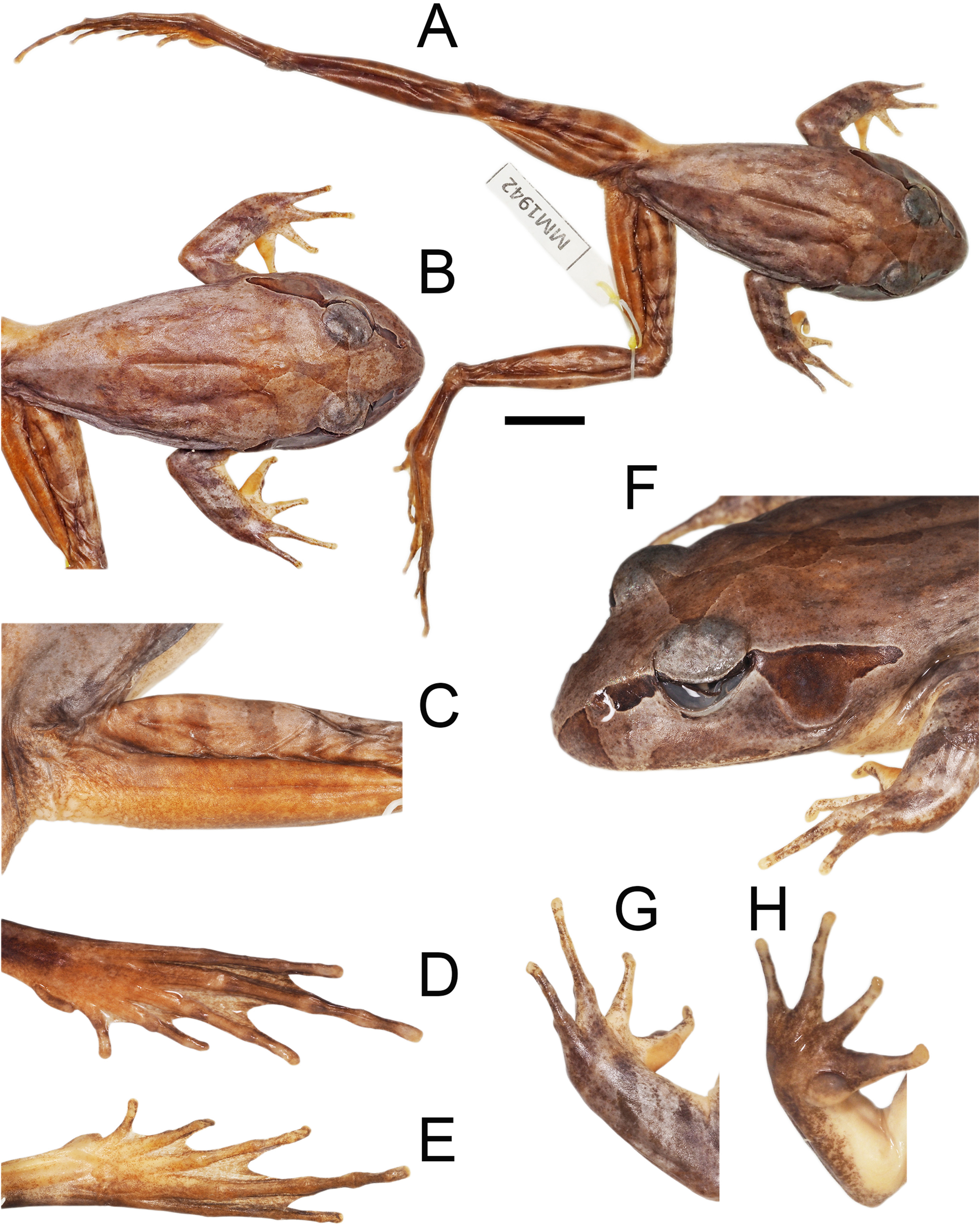
( Figs 6 View FIGURE 6 , 8 View FIGURE 8 , 12 View FIGURE 12 )
Holotype. AMS R.188750 ( ABTC 141374 View Materials ), adult male from Sharpes Creek , Gloucester Tops National Park, New South Wales, Australia, 32° 05’ S, 151° 68’ E, collected by Michael Mahony and Ross Knowles on 15 December 2015.
Material examined. see Supplementary Table S1 View TABLE 1 .
Diagnosis. Mixophyes australis sp. nov. is distinguished from M. hihihorlo by the presence of a dark triangular patch on the upper lip in front of the nostril with its apex at the nostril (vs absence), and absence of an uninterrupted narrow vertebral stripe extending from between the eyes to just above the vent (vs. presence); from M. iteratus and M. carbinensis by absence of black colouration with numerous small rounded pale spots on the posterior surface of the thigh; from M. coggeri by having posterior of thigh uniform (vs. with irregular pale blotches); from M. schevilli by having a discontinuous (vs. continuous) mid-dorsal stripe; from M. fleayi and M. fasciolatus by the lack of prominent black spots on the flanks and further from M. fasciolatus by the occurrence of pigmented patches on maxilla; from M. balbus by the occurrence of up to ten thin dark cross bands on the thighs (vs. five wider and more diffusely pigmented cross bands in M. balbus ); male advertisement call comprises one to four phrases with the mean number of notes in the first and second phrase fewer than in M. balbus (mean 7.24 vs. 12.24) with fewer double pulses in these phrases; and with a lower dominant frequency in both the advertisement (752 Hz vs. 773 Hz) and encounter (704 Hz vs. 788 Hz) calls.
In the field, M. australis sp. nov. is most likely to be confused with specimens of M. fasciolatus and M. iteratus which occur sympatrically. In addition to the features listed above, M. australis sp. nov. can be distinguished from M. iteratus by less extensive webbing of the toes that reaches to the distal tubercle of the fourth toe in M. iteratus . From M. fasciolatus it can be distinguished by the occurrence of darker markings on the lateral margins of the maxilla and mandible, in M. fasciolatus the maxilla is unmarked and is lighter coloured than the general background producing a distinctive paler area; and the soles of the feet are not pigmented black versus pigmented brown to black in M. fasciolatus .
Dimensions of holotype (in mm). SVL , 67; TL, 39.6; HL, 26.9; HW, 26.2; EN, 4.9; IND, 7.1; ED, 8.1; IOD, 12.8; TD, 5.9; FLL, 16.7; Fin3L, 16.1; MT, 4.8.
Description of holotype. Head flattened, snout prominent, gently rounded when viewed from above and in profile; nostrils more lateral than superior; canthus rostralis well defined, slightly concave; loreal region nearly flat, inclined laterally ( Fig. 12 View FIGURE 12 ). Eye relatively large (ED/EN = 1.67), pupil vertical when constricted; eye to naris span longer than internarial span (EN/IND = 0.69); tympanum large, nearly three-quarters eye diameter (TD/ED = 0.73), conspicuous, oval with vertical long axis. Vomerine teeth relatively long, oblique rows angled from anterior margin of choanae to midline between choanae; tongue approximately rectangular, not notched posteriorly.
Fingers robust, unwebbed. Subarticular and palmar tubercles prominent; relative length of fingers 3>2=4=1.
Hind limbs long (TL/SVL = 0.59); toes moderately webbed, on outer side of toes webbing reaches penultimate sub-articular tubercle on toes 1, 2 and 3, and second tubercle on toe 4, all toes with lateral flanges extending along toes to the terminal disc; tips of toes slightly expanded but not forming terminal disc ( Fig. 12 View FIGURE 12 ). Subarticular tubercles prominent, oval and conical; outer metatarsal tubercle absent; inner metatarsal tubercle prominent, slightly curved, with well-developed outer edge, approximately equal to the length of toe 1, not sharp or keratinized pigmented same as ventral surface of foot; relative length of toes 4>5>3>2>1.
Dorsum finely granular with small tubercules, venter smooth. Series of raised conical tubercles on ventral surface of thigh form a triangular area in pelvic patch with the tubercles becoming larger toward the mid-line and cloaca. Several large conical tubercles around the cloaca opening.
Variation. SVL of males 52–69 mm and females 64–81 mm ( Table 5). Females are larger than males, being an average of 1.3 times the SVL of males ( Table 5) but are otherwise similar in body proportions ( Table 5), colour in preservative, and patterning.
Hind limbs long (TL/SVL = 0.62 ± 0.03, range 0.56–0.70). Head approximately as long as broad (HL/HW = 0.92±0.06, range 0.77–1.06; eye diameter greater than eye to naris distance (ED/EN = 1.53±0.19, range 1.08–1.98), and eye to naris distance versus internarial span variable (EN/IND = 0.8 ± 0.1, range 0.62–1.16). Ventral surface is smooth. Brown glandular nuptial pad on first and second finger and inner palmar tubercle in breeding males.
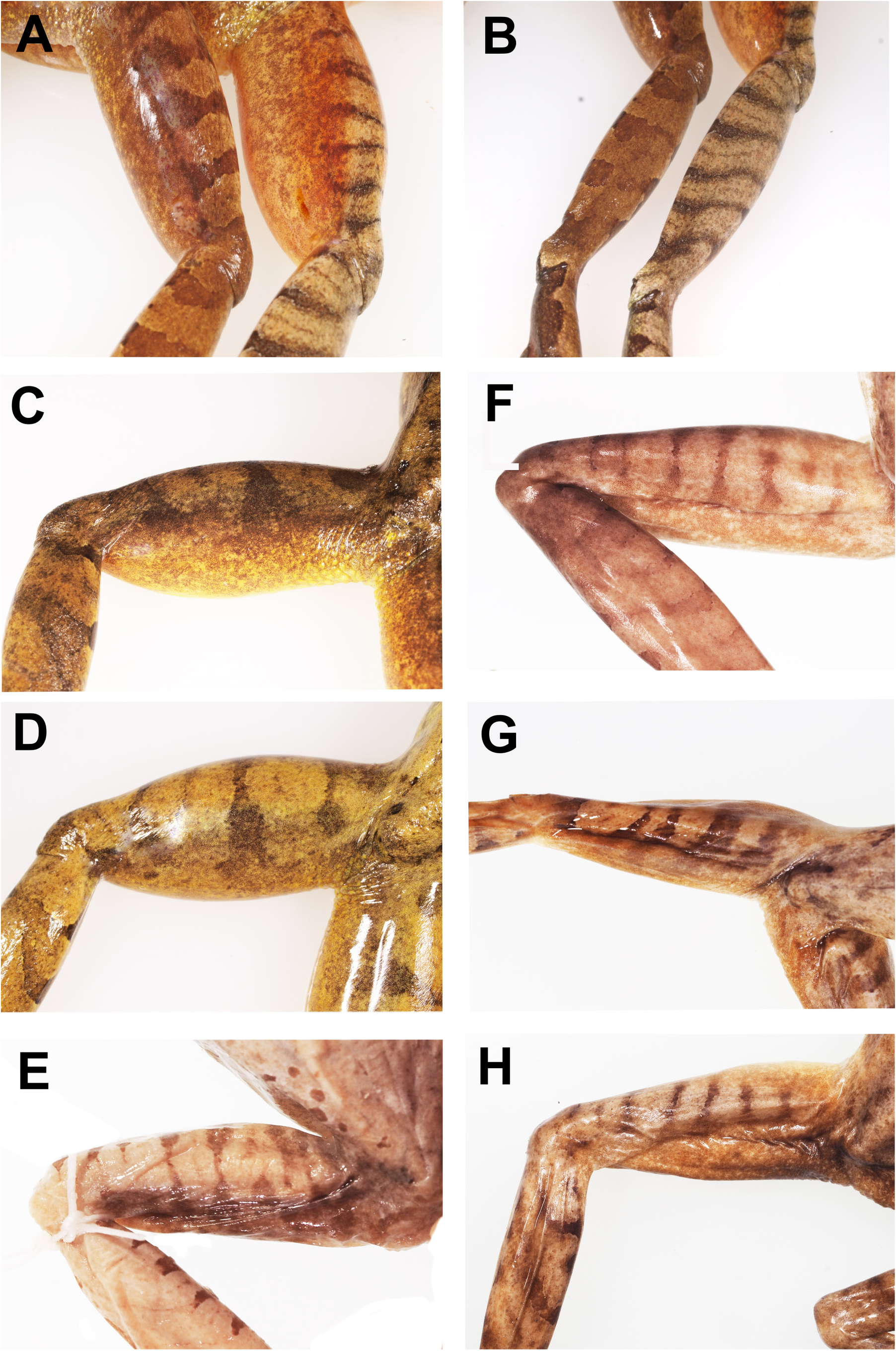
Colour in life. Dorsal surfaces rich copper to tan or dark brown or with green tinge, sometimes with burnt orange wash towards groin; a posteriorly oriented inter-orbital bar that extends as a mid-dorsal stripe, finely edged in black and highly variable in length and shape with uneven edges, often not continuous and usually terminating on the upper back but occasional extending to the mid-sacral region; remainder of dorsal surface either sparsely flecked with small black spots or a few larger blotches or a combination of both ( Fig. 8 View FIGURE 8 ). A dark triangular patch on either side of the snout with the apex at the external nostril but does not include the immediate area around the nostril. A dark lateral head-stripe extends from slightly posterior of the nostril, through eye, curves over tympanum, and extends posteriorly down anterior margin of the tympanum, sometimes including upper third of the tympanum, terminating above axilla; width variable but in all cases posterior margin is widest; bordered above and below by fine cream or fawn line that is sharply demarcated against the dark stripe. Loreal region is light coloured with darker patches along the upper jaw (maxilla).
Lateral surface and groin flushed with a reddish tan or peach, without spots or blotches, these surfaces noticeably lighter than dorsum, with colour diffusing gradually into the ventral colouration. Venter including throat and ventral surface of thighs immaculate white, cream or with a light lemon-yellow wash; lower jaw edged with diffuse brown wash.
In adults, iris dark brown merging into golden brown above pupil with an iridescent pale blue dorsal crescent.
Upper surfaces of the legs, feet, arms and hands with dark transverse cross bars, not extending on to posterior thighs; posterior surface of thighs uniform brown to burnt orange brown and slightly mottled; unpigmented triangular area with distinct closely grouped tubercles beneath the cloaca; anterior lateral edge of shank, posterior lateral edges of arms, and plantar surface of feet and tarsus dark brown; lateral palmar surfaces of outer fingers dark.
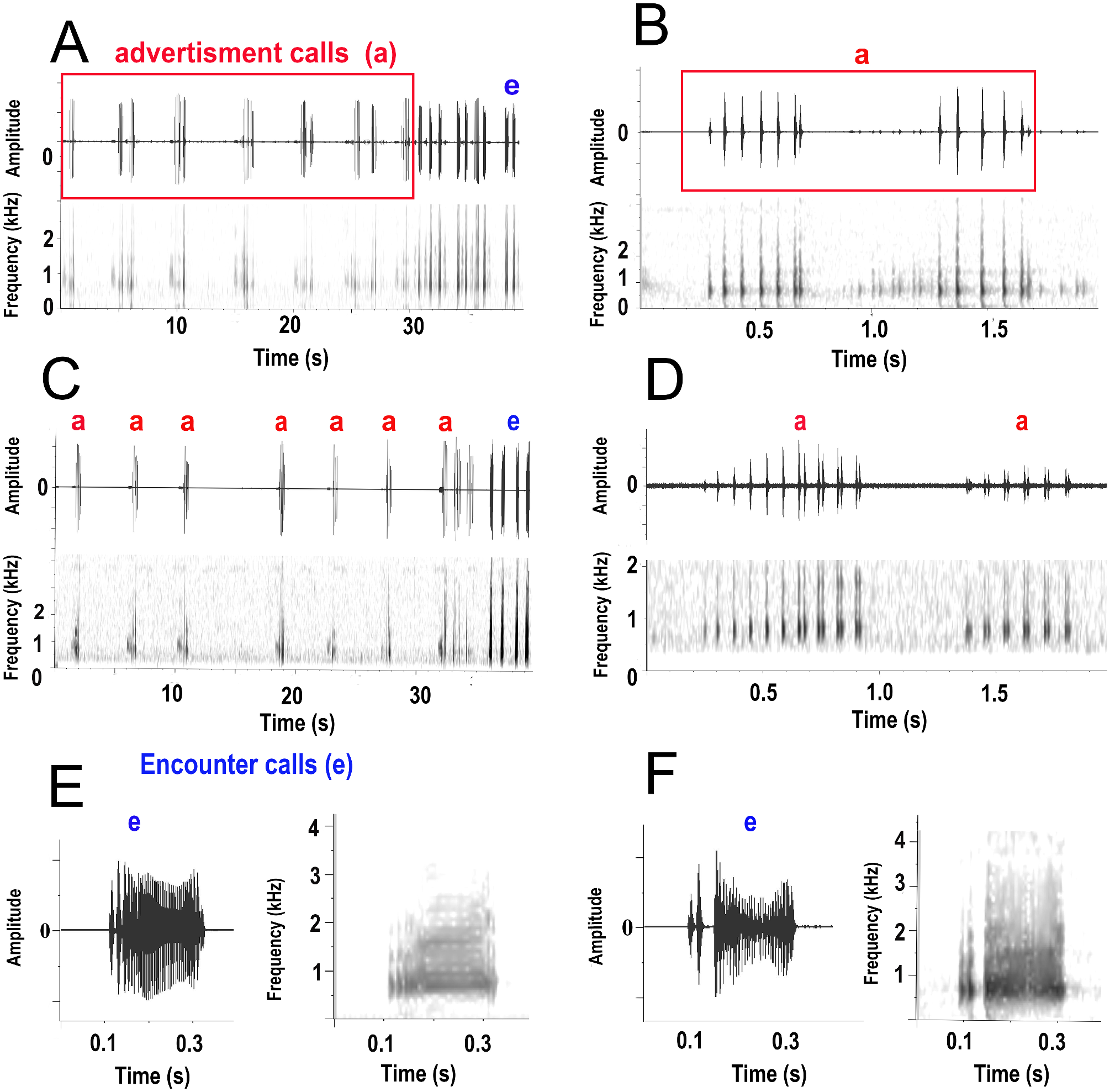
Reproductive call. The male advertisement call comprises one to four short notes repeated in rapid succession. Because of the variation in the number of notes in the call we provide means and ranges for measurements of call traits for the first two notes. Mean duration for a two note call is 1.34 s (range 1.29–1.35 s). There are commonly two notes in the call with the first of longer duration than the second (mean 0.69 and 0.45 s, ranges 0.30 0.70, and 0.18–0.45 respectively), with a short inter-note interval (mean 0.36 s), and with more pulses in the first note than the second (mean 8.6, and 6.2, ranges 5–11, 3–8 respectively). Pulse duration is much shorter (mean 0.015 s, range 0.011–0.017) than inter-pulse interval (0.083 s, range 0.080–0.088). Pulse structure varies within notes, with single pulses produced initially, followed by a series of pulses produced in couplets ( Table 2, Fig. 9 View FIGURE 9 ). The second note has more couplets than the first note ( Table 2, Fig. 9 View FIGURE 9 ). Pulse repetition rate is lower in the first note than the second (15.95 and 20.86 respectively) ( Table 2). Mean inter-call interval is 4 s (range 3–5 s) and males usually produce a series of calls before a period in which all calling ceases. Calling occurs during the spring and summer seasons when climatic conditions are suitable (MM pers. obs. Details of suitable conditions not reported here). Within the calling season, calling is initiated by a male, and this stimulates calling in nearby males and a chorus of calls with a short lag time or small overlap between the call of one male and a nearby male occurs. The male encounter call is a single note of moderate duration (mean 0.23 s, range 0.24–0.48 s) composed of dense pulses and is often uttered among advertisement calls and males may produce several encounter calls in succession ( Fig. 9C View FIGURE 9 ). The dominant frequency of the notes in the advertisement call differs slightly with the first note having a higher frequency than the second (732 and 742 Hz, range 650 to 768Hz). The DF of the encounter call (704 Hz, range 632 to 831Hz) is slightly lower than the advertisement call. Advertisement and encounter calls are not frequency modulated. Call recordings have been deposited at the Australian Museum as a multimedia record attached to the database record for the holotype R.188750.
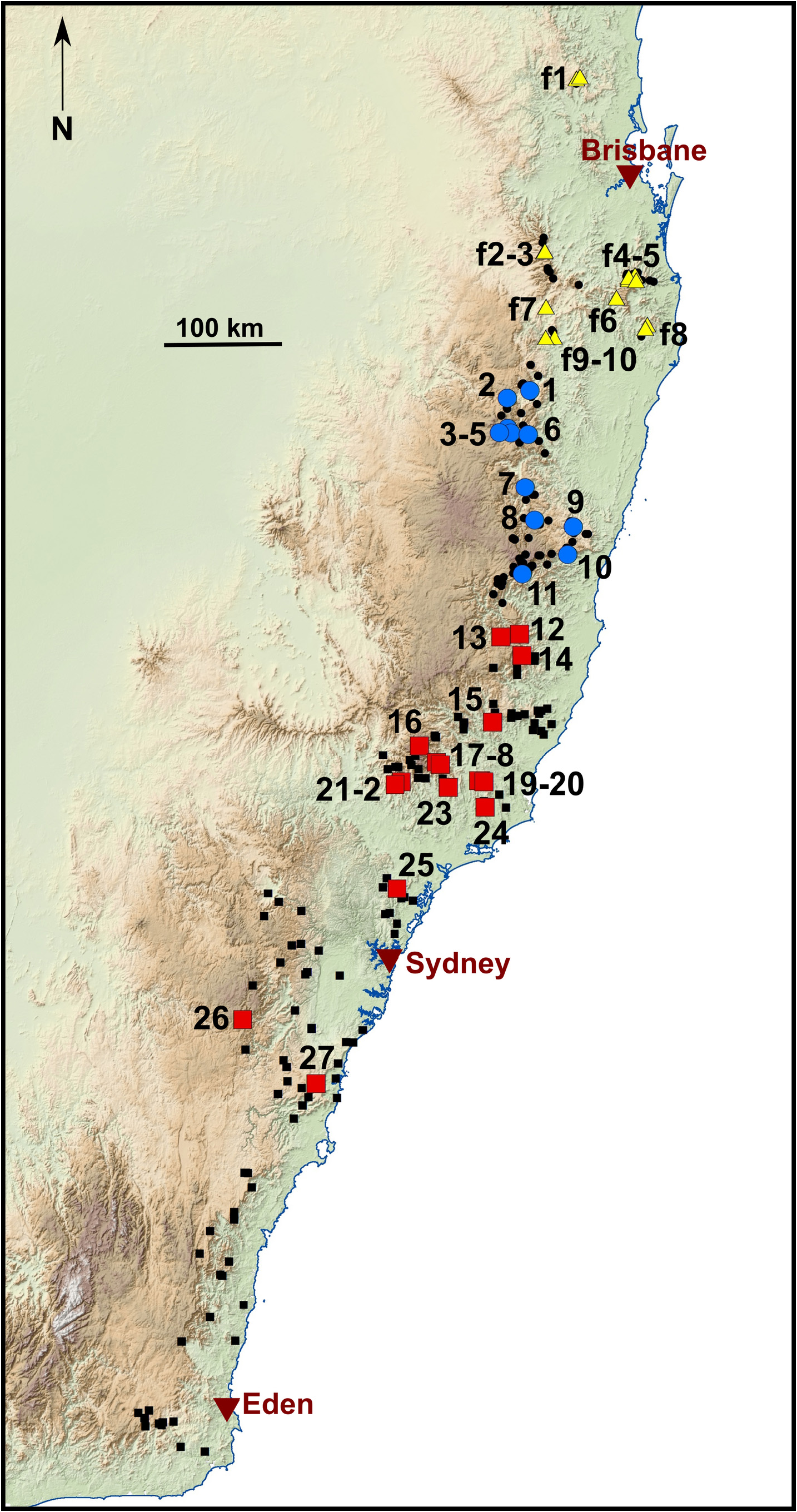
Distribution. Mixophyes australis sp. nov. is distributed from the Carrai Plateau on the southern side of the Macleay River in New South Wales south to the Cann River catchment in East Gippsland Victoria ( Fig. 1 View FIGURE 1 ).All records are from wet forest habitats in drainages that flow to the east of the Great Dividing Range. Its distribution includes three bioregions ( Thackway & Cresswell 1995), the southern quarter of the North East Coast, the Sydney Basin and Southeast Corner bioregions. This species occurs across almost all available elevations in these bioregions. For example, in the Barrington Range it occurs from 390 m asl (Sharpes Creek) up to 1230 m asl (Dilgry River), and in the Sydney Basin from 50 m asl (Stanwell Tops) up to 1000 m asl (Blackheath, Mount Irvine and Mount Wilson). In the Southeast Corner bioregion, the highest altitude record is 810 m asl (Bombala), and the southernmost location, at Chandlers Creek, East Gippsland, is at 280 m asl.
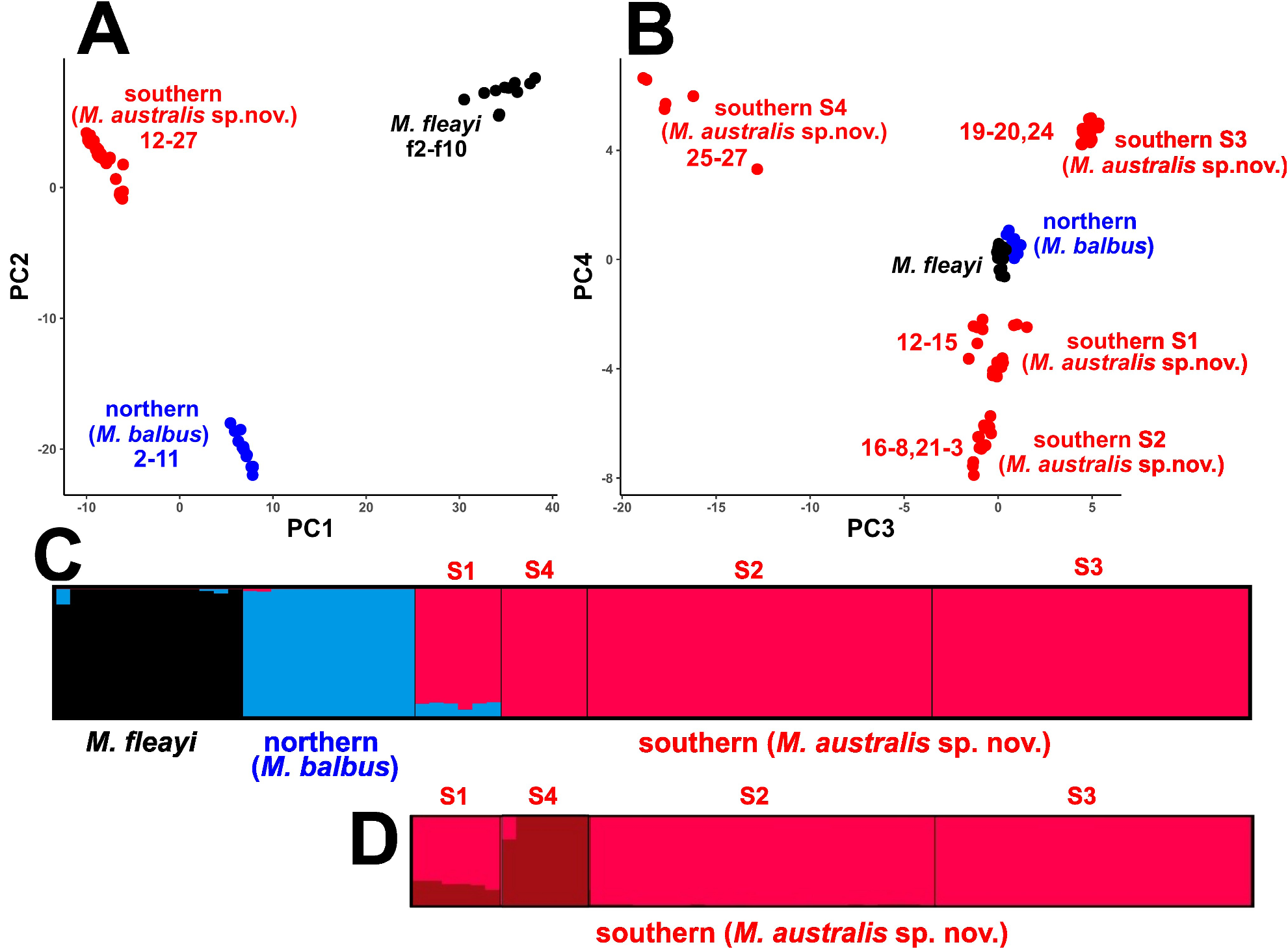
We note that genetic analysis of southern NSW and north-eastern Victorian populations of Mixophyes from spirit preserved vouchers should be considered. This region has a little appreciated biogeographic barrier immediately to the north of Nowra, which in some recent studies on frogs is manifested as a deep species level break that also may have an influence as a barrier to north–south movement more widely on the coastal fauna of southern coastal NSW and north-eastern Victoria ( Mahony et al. 2020, 2021).
Habitat and Ecology. The ecological requirements of adults and larvae of M. australis sp. nov. are reasonably well known. This species is found in association with first and second order permanent and ephemeral streams in temperate and sub-tropical rainforest, wet sclerophyll forest, and also in moist gullies in dry forest ( Gillespie & Hines 1999). Statistical modelling using presence and absence data and 24 environmental predictors from the northern portion of the range of M. australis sp. nov. (Barrington and Hastings Ranges) showed a preference for the interiors of large forest tracts in areas with relatively cool mean annual temperatures, at sites that were typically free from any disturbance with a thick canopy and relatively simple understorey ( NSW NEFBS 1994).
Adult males spend most of their lives in the riparian zone where they shelter under leaf litter or burrow into the topsoil or under debris such as logs when inactive during all seasons. Males leave these protected sites during active periods of calling from spring through to autumn, at which times they call from stream banks or among rocks next to the riffle zone where oviposition occurs ( Knowles et al. 2015). Females move away from the riparian zone and, like males, shelter under leaf litter and burrow into the topsoil during periods of inactivity (Mahony pers. obs.). Larvae are nektonic and occupy pools between riffle zones in small streams ( Daly 1998).
Eggs and larvae. Embryonic development and larval morphology were described by Anstis (2013), from specimens collected at five locations that include three within the range of M. australis sp. nov. (Tirrill Creek, Bulga State Forest; Sharpes Creek, Barrington Range; and Gap Creek, Central Coast Range), and two within the range of M. balbus (near Point Lookout, New England Range, near type locality; Coombadjha Creek, Gibraltar Range). No morphological features that distinguished among these samples were reported ( Anstis 2013), but it is possible that variation was present that was not reported since Anstis was not aware of the presence of two species amongst her samples. Mixophyes australis sp. nov. deposit eggs among small pebbles or debris in gently flowing shallow water ( Knowles et al. 2015).
Etymology. The specific Latin epithet australis refers to the species being the most southerly distributed member of Mixophyes .
Assessment of IUCN threat category for M. australis sp. nov. We assess M. australis sp. nov. to be Endangered under category A2(a)B2(a)(b). There is an estimated population reduction of>30% over the past decade and the causes may not have ceased (Supplementary Text. Figs S4—S View FIGURE 4 6 View FIGURE 6 ). The Area of Occupancy (AOO) in the decade 2011-2020 is 188 km 2, which is well below the threshold level of 2,000 km 2, and there is evidence of a continuing population reduction based on mapping of database records over three generations (Supplementary Text Fig. S1 View FIGURE 1 ). Mixophyes australis sp. nov. is known over a relatively large range (EOO for all records is a convex hull of 81,578 km 2, and an alpha-hull of 2,734 km 2). In the decade from 2001-2010, these values reduced to 33,003 km 2 and 1,495 km 2 respectively, which demonstrates the rate of contraction of the distribution. Mapping of the records reveals declines and disappearances of populations in the southern two thirds of the distribution (Supplementary Text Fig.S1 View FIGURE 1 ). Gillespie et al. (2014) reviewed the conservation status of the Victorian and southern NSW populations and noted that prior to 1983 only five specimens had been recorded in Victoria from three localities. To our knowledge there have been no observations of this species in Victoria in the past two decades. Field surveys in the south-eastern forest region and the Greater Blue Mountains area of NSW conducted in the past decade have documented the loss of populations in this large region ( Daly & Craven 2011, Mahony unpubl. data). Fortunately, this decline has occurred only in the southern two thirds of the range and intensive surveys in the northern third including the Central Coast, Myall, Barrington and Hasting Ranges show that the species remains in these locations (this study, see Supplementary Text) and that populations have remained stable over the past decade ( Mahony 2007, 2013).
Because genetic evidence indicates that there are two subpopulations of M. australis sp. nov. that are separated by the Hunter River valley, a northern subpopulation equivalent to genetic clusters S1, S2 and S3, and a southern subpopulation equivalent to cluster S4, we also assess their threat status (Supplementary Text). The northern subpopulation (i.e., Myall Range, Barrington Range and Hastings Range), and the southern subpopulation (Central Coast Range, Blue Mountains and Illawarra region to east Gippsland) meet the conservation status assessment criteria for Endangered 2B1a,b (Supplementary Text Table S1 View TABLE 1 ).
Threats considered to impact M. australis sp. nov. are the same as those for M. balbus but the extensive contraction of the species’ distribution in the southern portion of its former range is a matter of considerable conservation concern. The recovery of many populations in the northern third of its range provides an option for the return of this frog to the southern part of its former distribution. In support of such an action, successful captive husbandry of Mixophyes has been reported ( Banks et al. 2014) and protocols for translocations and reintroduction for Bd affected species are available ( Scheele et al. 2021). While the northern populations may provide frogs for reintroduction, it should be noted that northern and southern populations are genetically very divergent, and the northern populations occupy an environmental envelope where the organism x environment interaction could be more favourable to the frog than may occur in the southern part of the range. Given the above, a precautionary approach using an experimental evaluation of the survival of northern frogs in an environment that they have not experienced in their recent evolutionary history including a recent period of their exposure to Bd, may be an initial action.
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.