Microplana aixandrei, Vila-Farré, Miquel, Mateos, Eduardo, Sluys, Ronald & Romero, Rafael, 2008
|
publication ID |
https://doi.org/ 10.5281/zenodo.181468 |
|
DOI |
https://doi.org/10.5281/zenodo.5695776 |
|
persistent identifier |
https://treatment.plazi.org/id/03D287BA-FFFE-D31C-85C7-F8B4FD8CF967 |
|
treatment provided by |
Plazi |
|
scientific name |
Microplana aixandrei |
| status |
sp. nov. |
Microplana aixandrei sp. nov.
Material. Holotype, CRBA 435, Llano del Berral (lat. 36.75428, long. -5.45399; alt. approx. 657 m) in the central sector of the Sierra de Grazalema, Cádiz ( Spain), 5 December 2004, sagittal sections on 1 slide.
Paratypes: CRBA 436, ibid., sagittal section on 1 slide; CRBA 437, ibid., horizontal sections on 1 slide.
Diagnosis. Microplana aixandrei sp. nov. can be distinguished from its congeners by its small size (up to 10 mm long), cylindrical body tapering anteriorly to a blunt point, bluntly pointed tail, and hyaline body surface. Regarding anatomical features, the species differs from its congeners in the following features: presence of two ventral testes on either side of the body; spherical penis bulb provided with a strong musculature and a distinct bulbar lumen; short and vertically oriented penis papilla; atrium divided in a cup-shaped cavity and a tubular distal cavity; wide and obliquely orientated bursal canal with a sphincter at its proximal end; a copulatory bursa without genito-intestinal connections; use of spermatophore in the transfer of sperm.
Ecology and distribution. The species is known only from the type locality. In contrast to other Iberian terrestrial planarians, M. aixandrei can be considered a relatively common species of the soil fauna at the type locality. During mating the sperm is transferred to the copulatory bursa of the partner aggregated in a spermatophore.
Etymology. The specific epithet is based on the nickname of Vila’s grandfather, Miquel Farré Servent, who lived in a house known as “casa l’Aixandre” in his hometown Salàs del Pallars.
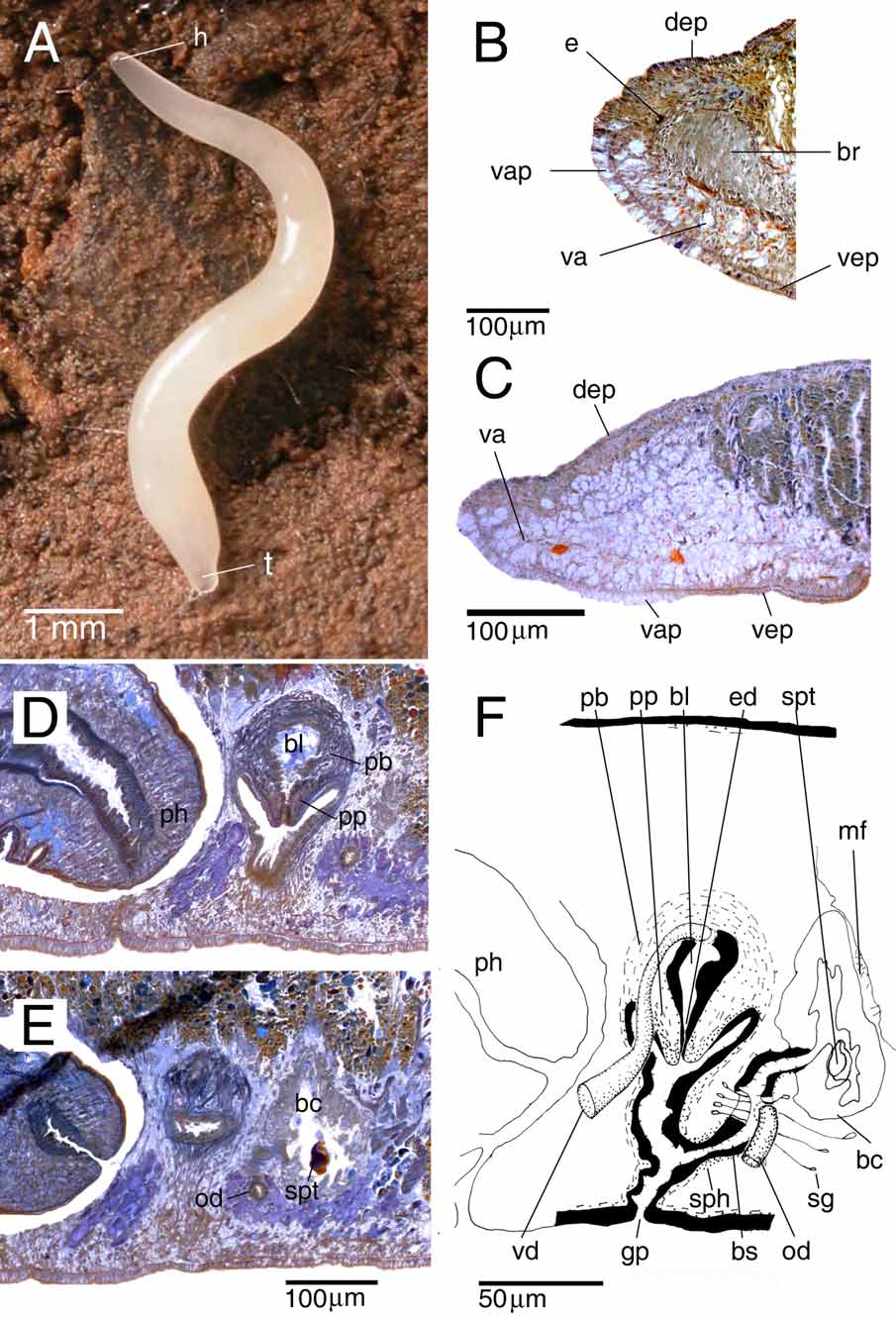
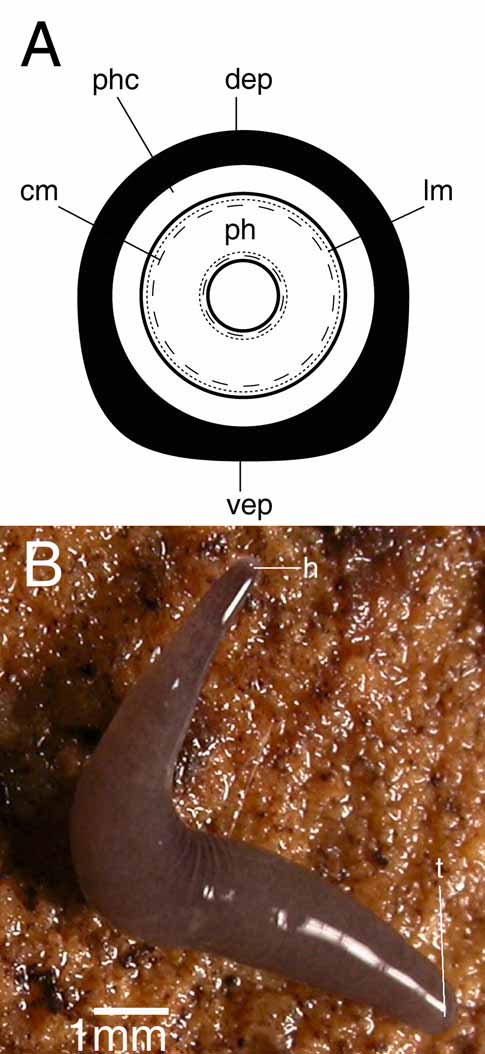
Description. The living, sexually mature specimen measured 10 mm in length and about 0.5 mm in width, in elongated state ( Fig. 2 View FIGURE 2 A). The cylindrical body tapers anteriorly to a blunt point; tail also bluntly pointed. The body surface is hyaline and therefore the species appears white in colour due to the content of the intestine. The anterior and posterior ends, where the intestine is absent, are transparent. The ventral surface is hyaline. The hyaline colouration and the tiny size of the preserved specimens prevented us to adequately observe the creeping sole.
The two small eyes (eye cup diameter 11 µm in sections) are located at a short distance in front of the brain ( Fig. 2 View FIGURE 2 B). In living and preserved specimens the eyes are only clearly visible under observation through a dissecting microscope.
The anterior body region is filled with vacuolated parenchymal tissue ( Fig. 2 View FIGURE 2 B, C) that is reduced between the eyes. This region with vacuolated tissue extends backwards to the level of the testes. The adjacent ventral epidermis is also vacuolated and thick (about 25–30 µm in longitudinal sections while approximately 18 µm in the adjacent non-vacuolated ventral and dorsal epidermis); this vacuolated part of the epidermis ( Fig. 2 View FIGURE 2 C) extends to almost the level of the ovaries.
The subepidermal longitudinal fibres of the body musculature are weak. In the ventral body region numerous longitudinal fibres are distributed in two parenchymal bands, which are especially strong over and under the ventral nerve cords. The scarce dorsal longitudinal parenchymal fibres are very weak and apparently discontinuous.
The cylindrical pharynx is about one-eighth of the body-length (0.3 mm) and is situated in the posterior third of the animal, in an almost horizontal position. The outer epithelium, which is ciliated only at the posterior part of the pharynx, is underlain by a layer of longitudinal muscles, followed by a layer of circular muscles ( Fig. 3A View FIGURE 3. A ), intermingled with some additional longitudinal fibres (not represented in Fig. 3A View FIGURE 3. A ). Close to the dorsal insertion of the pharynx, the inner epithelium is underlain by a thin layer of circular muscles that becomes thicker at the posterior end of the pharynx. At the tip of the pharynx this circular layer narrows again before reaching the lumen. This circular layer is followed by a thick layer of longitudinal muscles. The mouth is situated at the posterior portion of the pharyngeal pocket, close to the hind wall of the pharyngeal pouch. In specimen CRBA435 the mouth is situated at 1.63 mm from the tip of the body and 0.15 mm from the gonopore.
There are two pairs of ventrally located, oblong testes follicles, occupying about one-fourth of the dorsoventral diameter in the prepharyngeal part of the body. The testes are located in the posterior third of the anterior body region. In specimen CRBA435 there is an additional immature third testis situated on one side of the animal.
The strongly muscular, spherical penis bulb is covered with intermingled longitudinal and circular muscle fibres and is provided with a well-developed bulbar lumen ( Fig. 2 View FIGURE 2 D). This bulbar lumen tapers gradually to form an ejaculatory duct that opens at the tip of the penis papilla. The bulbar lumen and ejaculatory duct are lined with a nucleated epithelium that is underlain with a layer of circular muscle fibres. At the level of the posterior section of the pharyngeal pocket the thin vasa deferentia (diameter about 6 μm at the mid-level of the pharyngeal pocket) enlarge to form spermiducal vesicles, which narrow before entering the penis bulb. After having penetrated the penis bulb, the vasa deferentia increase in diameter, subsequently opening separately into the dorsal part of the seminal vesicle.
The vertically oriented penis papilla is short and conical. The papilla is covered with a thin, nucleated epithelium that is underlain with a thick, subepithelial layer of circular muscle bound by a layer of longitudinal fibres.
The atrium consists of a dorsal cup-shaped cavity and a distal tubular part. The lining epithelium of the atrium is underlain with a subepithelial circular muscle layer, thickened at the posterior wall of the tubular part, followed by a thin layer of longitudinal muscles.
The ovaries are situated immediately above the ventral nerve cords. They lie at about one-third of the distance between the anterior end of the body and the root of the pharynx, occupying about one–fifth of the dorso-ventral diameter. The oviducts arise from the ventral side of the ovaries. In running backwards, the ducts follow the course of the ventral nerve cords. Behind the gonopore the ducts turn dorsally and open separately into the bursal canal.
The copulatory bursa is an irregular sac-shaped structure, lined with tall vacuolated cells ( Fig. 2 View FIGURE 2 E). Several muscle fibres traverse in dorso-ventral direction the parenchyma between the posterior wall of the copulatory bursa and the adjacent intestinal branch ( Fig. 2 View FIGURE 2 F). The copulatory bursa is not connected with the gut.
All three of the animals examined had in their copulatory bursa remnants of an irregular structure (37x21 µm in specimen CRBA435) formed by a blue homogeneous substance partially enveloped by a thin, brown layer, most likely of sclerotic nature ( Fig. 2 View FIGURE 2 E). We have not found the origin of this substance in penial or atrial glands, but the colour and texture resemble the wall of a cocoon capsule. The location and nature of this structure suggest that it forms part of a spermatophore.
The wide bursal canal, which receives the opening of the shell glands at the same level as it receives the oviducts, is lined with nucleated cells. The distal section of the oviducts, just before communicating with the bursal canal, also receives the secretion of the shell glands. The bursal canal is surrounded by a subepithelial layer of circular muscles and some scattered longitudinal muscles fibres. A sphincter, consisting of circular muscle fibres, surrounds the proximal section of the bursal canal ( Fig. 2 View FIGURE 2 F).
Discussion. Among the approximately 19 species of native land planarians known from Europe, three of which are newly reported in the present paper, M. aixandrei stands apart from the other species by a combination of external features and the anatomy of its genital apparatus.
A hyaline body colouration is also found in M humicola Vedjovsky, 1890 . However, this species shows a greenish anterior end, in contrast to M. aixandrei . Regarding anatomical features, M. humicola has dorsal testes and a genito-intestinal duct, while a copulatory bursa is absent ( Schneider, 1935). In contrast, M. aixandrei presents ventral testes and a copulatory bursa, but lacks a genito-intestinal duct.
A copulatory bursa that is devoid of any connection with the intestine occurs also in M. mahnerti Minelli, 1977 , M. styriaca ( Freisling, 1935) , and M. grazalemica sp. nov. described below.
Microplana mahnerti shows about twenty testes on each side of the body and an elongated penis papilla. In contrast, M. aixandrei shows two testes on each side of the body and a short and conical penis papilla. In M. mahnerti the bursal canal runs from the wall of the atrium parallel to the body surface and the oviducts open at its distal section. A sphincter is absent ( Minelli, 1977). In contrast, in M. aixandrei the bursal canal is a obliquely running structure that receives the oviducts at its central region, while a sphincter is present in the proximal section of the canal. With respect to external features, M. mahnerti shows a grey colouration and big size (13 mm in preserved specimens), contrasting with the hyaline colouration and reduced size of M. aixandrei (about 4 mm in preserved specimens).
In M. styriaca ( Freisling, 1935) an expanded bulbar lumen is absent, while the penis bulb and papilla are elongated. In contrast, in M. aixandrei a distinct bulbar lumen is present, while the penis bulb is rounded and the penis papilla is short and conical. In M. styriaca the oviducts form a short common oviduct before they enter the bursal canal ( Freisling, 1935), contrasting with the oviducts in M. aixandrei that open separately into the bursal canal. Furthermore, M styriaca is dark-brown, whereas M. aixandrei is hyaline.
The new species M. grazalemica sp. nov. is not hyaline, contrasting with the hyaline colouration of M. aixandrei . With respect to anatomical features, M. grazalemica possesses about 15 testes on each side of the body, in contrast to the two pairs present in M. aixandrei . The presence of a spermatophore in the copulatory bursa of the three specimens of M. aixandrei suggests that the animals are adult worms and not juveniles of M. grazalemica sp. nov. The use of spermatophores represents a unique feature of this species, not previously recorded in any species of the Microplaninae. A vacuolated parenchymal tissue in the anterior part of the body is absent in M. grazalemica but present in M. aixandrei .
There are various explanations for the vacuolation of the epidermis and parenchyma observed in M. aixandrei : vacuolation as an artefact of tissue fixation, processing and subsequent histological preparative treatment, apparent vacuolation due to the chromophobicity of the contents of the vacuoles, normally vacuolated planarian tissues, or vacuolation due to a disease process.
A heavy infection of gregarine parasites can result in pathological peri-intestinal histolytic vacuolation of the mesenchyme. In this condition the epidermis is not involved; rather gregarine gamonts and zygocysts are present in the parenchyma surrounding the gut trunk, branches and diverticula, and gamonts are generally present in the gut lumen and mesenchyme (L. Winsor, personal communication). No gregarines were present in the specimens of M. aixandrei examined here, and both epidermis and parenchyma exhibit vacuolation.
Normally vacuolated and vesicular tissues in planarians are associated with the reproductive organs, such as the phagocytic cells of sperm resorptive tissues in various bursae, vitelline follicles, ovarian tubae, and parovarian tissues ( Cernosvitov, 1931; Sluys, 1989b; Winsor, 2006). The vaculoate epidermis and parenchyma in M. aixandrei are located at the anterior tip and are not associated with reproductive structures. Nor do they exhibit the fine cytological characteristics of resorptive tissues.
In addition to the routine oversight stain, sections were stained with the alcian blue-periodic acid-Schiff (AB-PAS) technique to detect hexose-containing and sialic acid-containing mucosubstances, and alcianophilic carbohydrates with carboxylated and sulphate ester groups, which in the adhesive musculo-glandular organ in the terricolan Pimea can be weakly basiphil to chromophobic ( Winsor, 1991). The AB-PAS gave negative results. It is, therefore, concluded that nothing is present in the vacuoles. The most likely explanation is that something has been lost during fixation or processing. Strongly acidic fixatives such as Heidenhain’s SUSA and Bouin’s dissolve or fail to stabilize certain secretory elements such as types of acidophil or “zymogen” granules ( Drury & Wallington, 1980; Leal-Zanchet & Hauser, 1999). Nitric acid is a protein-coagulant fixative, a suitable concentration in a fixative for which is 0.5M ( Baker, 1958). However the concentration of nitric acid in Steinmann’s fixative is approximately 5M, making the reagent very strongly acidic. When additional specimens of M. aixandrei are available they will be fixed in non-acid and formaldehyde-based fixatives for further histochemical studies.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Microplana aixandrei
| Vila-Farré, Miquel, Mateos, Eduardo, Sluys, Ronald & Romero, Rafael 2008 |
M. styriaca (
| Freisling 1935 |