Martes martes ( Linnaeus, 1758 )
|
publication ID |
https://doi.org/ 10.1093/mspecies/seac007 |
|
publication LSID |
lsid:zoobank.org:pub:A0366165-B2C1-47E6-95ED-E99CBE8AA7A4 |
|
persistent identifier |
https://treatment.plazi.org/id/03D18052-705A-FFC7-FC29-FBE26D9D9694 |
|
treatment provided by |
Felipe |
|
scientific name |
Martes martes ( Linnaeus, 1758 ) |
| status |
|
Martes martes ( Linnaeus, 1758) View in CoL
European Pine Marten
Mustela martes Linnaeus, 1758:46 View in CoL View Cited Treatment . Type locality “sylvis antiquis;” restricted to “ Upsala,” Sweden, by Thomas (1911:139).
Mustela sylvestris Oken, 1816:1029 View in CoL . Unavailable name ( International Commission on Zoological Nomenclature 1956; Opinion 417).
Martes vulgaris Griffith, 1827:123 View in CoL . Type locality “Northern parts of Europe and Great Britain.”
Martes sylvatica Nilsson, 1847:41 View in CoL . Type locality “Scandinavia.”
Martes abietum Gray, 1865:104 View in CoL . Type locality “Europe, England and France, Russia.”
Martes martes latinorum Barrett-Hamilton, 1904:389 View in CoL . Type locality “ Sardinia Isle,” Italy.
Martes martes var. notialis Cavazza, 1912 View in CoL [1911]:181. Type locality “southern Italy.”
Martes martes lorenzi Ognev, 1926:47 View in CoL . Type locality “Storozhevaya (vicinity),” Kuban province, Russia.
Martes martes ruthena Ognev, 1926:49 View in CoL . Type locality “Dmitrovsk vicinity,” Moscow province, Russia.
Martes martes ruthena View in CoL borealis Kuznetzov, 1941:126. Type locality “Shenkursk district,” Arkhangelsk province, Russia.
Martes martes uralensis Kuznetzov, 1941:126 View in CoL . Type locality
“Miass vicinity,” southern Urals, Russia.
Martes martes borealis : Bobrinskoy (in Bobrinskoy et al.) 1944:121). Name combination.
Martes martes sabaneevi Jurgenson, 1947:147 , 179. Type locality “Pechora River” (territory of Pechoro-Ilychskii Reserve), Northern Urals, Russia.
Martes martes kuznetsovi Pavlinov and Rossolimo, 1987:61 . Replacement name for Martes martes ruthena View in CoL borealis Kuznetsov, 1944.
Martes martes minoricensis Alcover, Delibes, Gosálbez, and Nadal, 1986:331 View in CoL . Type locality “exceptionally on the Menorca Isle,” Spain.
CONTEXT AND CONTENT. Order Carnivora, family Mustelidae View in CoL , subfamily Mustelinae View in CoL , tribe Mustelini , genus Martes View in CoL , subgenus Martes View in CoL ( Anderson 1970; Pavlinov and Rossolimo 1987; Wozencraft 2005). No subspecies are currently recognized ( Larivière and Jennings 2009; Burgin et al. 2020; see “Nomenclatural Notes”).
NOMENCLATURAL NOTES. Ten nomenclaturally valid subspecies, including the nominal martes , have been named in the scientific literature. Two to 10 subspecies have been recognized: two subspecies by Ognev (1931), five by Heptner et al. (1967, 2001) and Aristov and Baryshnikov (2001), six by Gromov et al. (1963), seven by Ellerman and Morrison-Scott (1951) and Grakov (1981), eight by Wozencraft (2005), nine by Abramov and Khlyap (2012), and 10 by Pavlinov and Rossolimo (1987). Given recent concern about the validity of subspecific taxonomy (e.g., Patton and Conroy 2017; Schiaffini 2020), an evaluation of subspecies of Martes martes with contemporary techniques and analyses is needed, which might be challenging because M. martes is considerably variable across its extensive geographic distribution ( Heptner et al. 1967, 2001; Grakov 1981; Larivière and Jennings 2009).
DIAGNOSIS
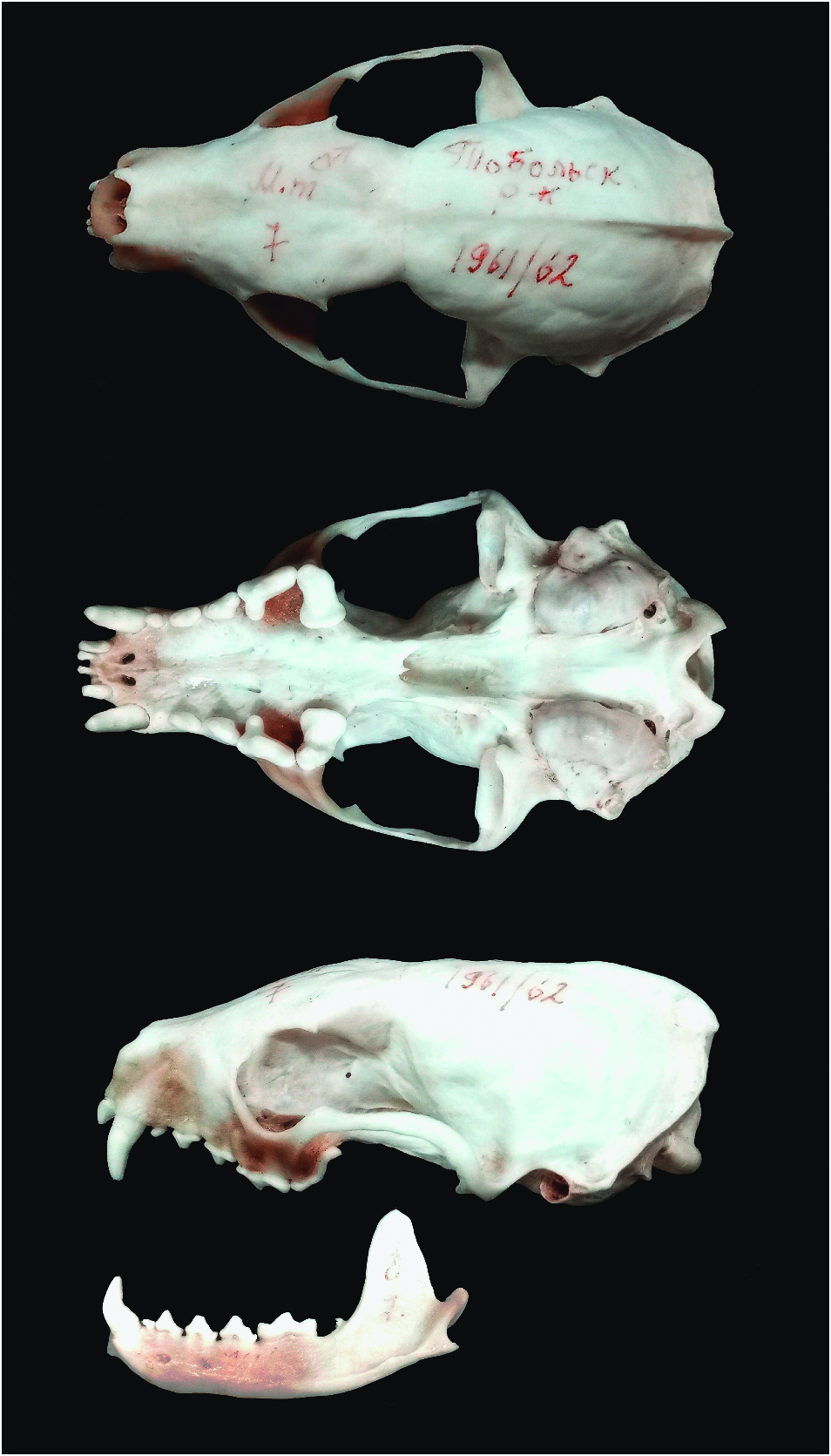
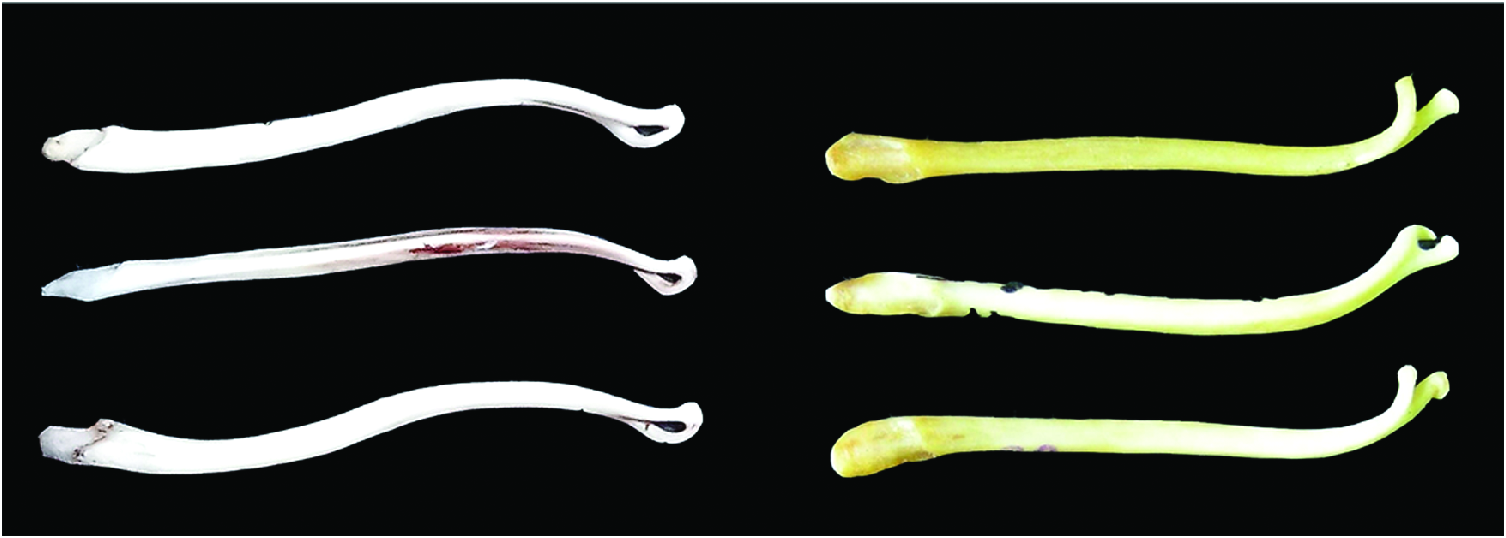
Martes martes is a small carnivorous mammal about the size of a domestic cat. It is very similar morphologically to M. zibellina (sable— Monakhov 2011), M. foina (stone marten— Stubbe 1993), M. americana (American marten— Clark et al. 1987), and M. melampus (Japanese marten— Hagmeier 1961; Anderson 1970). Pelage is lush and soft, without brilliance and grayness typical of M. zibellina . Coloration of M. martes is monotonous yellowish brown to pale yellow, rarely dark brown (Kusnetzov 1941; Stubbe 1993; Heptner et al. 2001). The head has the same color as the back, whereas in M. zibellina and M. melampus the head is lighter, and in M. flavigula (yellow-throated marten) and M. gwatkinsii (Nilgiri marten) it is darker. Paws are brown to black, finger-pads, unlike M. foina , are densely furred in winter, same as in M. zibellina , and claws are blonde. A yellow, or slightly orange, elongated “bib” almost always occurs on the neck and between and extending to the base of the front legs ( Fig. 1 View Fig ); it is rarely whitish (as in M. foina — Pavlinin 1963). Nose tip, unlike M. foina (flesh-colored), is black ( Görner and Hackethal 1988). The bushy tail is relatively long (> 19 cm in females and> 22 cm in males), measuring one-half to two-thirds of the body length, and approximately the same color as the back, with fluffy hair and a dull brown hue; the end is pointed ( Fig. 1 View Fig ), which differs from the shorter tail of M. zibellina (<17.2 cm in females and <19 cm in males; up to one-half of the body length— Aristov and Baryshnikov 2001) with its blunt, rounded end ( Novikov 1956). Several features of the skull can be used to distinguish M. martes from M. zibellina and M. foina . Compared to M. zibellina , M. martes has relatively elongated auditory bullae and there is a greater distance between the bullae; the processus mastoidei only slightly protrudes beyond the edges of the auditory foramen in M. martes ( Ognev 1931) . Oral edge of palatine arch, unlike M. zibellina , has a clear outgrowth in M. martes ( Pavlinin 1963) . Nasal bones unlike those of M. foina have a slight narrowing in the middle part ( Ognev 1931; Novikov 1956). Unlike M. foina , distance between mental foramina of M. martes is greater than diameter of lower canine ( Fig. 2 View Fig ; Novikov 1956); length of inner lobe of M1 is more than 70% of its width ( Monakhov 2011). The massive postorbital constriction of M. martes is proposed as distinguishable from the smaller postorbital constriction of M. foina ( Altuna 1973) and M. zibellina (Monakhov 2020) . Bacula of M. martes are 39–46 mm ( Aristov and Baryshnikov 2001), curved at the distal end ( Novikov 1956) with closed ring ( Fig. 3 View Fig ), which differs morphologically from M. zibellina (forked end forms an unclosed ring— Pavlinin 1963; Monakhov 2011). A key to the species of Martes can be found in Monakhov (2011).
GENERAL CHARACTERS
Martes martes is a small-sized, terrestrial, carnivore. It has an elongated body with a small triangular head. It is semiplantigrade and has relatively short, five-fingered paws: hindlegs are longer than the front legs. When walking and jumping, the back is arched up. Martes martes has marked sexual dimorphism: the male is 10–15% larger than the female ( Pavlinin 1959); therefore, size characteristics are given for males and females separately.
Body masses (kg) of males and females, respectively, were: 1.2–2.4 and 0.8–1.4 ( Görner and Hackethal 1988), 1.18–1.78 and 0.97–1.33 ( Stubbe 1993), 1.14–1.89 and 0.84–1.33 ( Ansorge 1988), 1.0–1.6 and 0.73–1.1 ( Danilov and Tumanov 1976), 0.76–1.63 and 0.56–1.1 ( Polezhaev 1998), and 1.13–1.94 and 0.88–1.44 ( Stier 2012). Head–body lengths (cm) for males and females, respectively, were: 44.5–49.0 and 42.0–50.0 ( Stubbe 1993), 42.0–52.2 and 41.0–49.0 ( Ansorge 1988), 39.5–57.0 and 37.5–44.0 ( Danilov and Tumanov 1976), 38.0–46.7 and 34.5– 42.0 ( Polezhaev 1998), and 38.9–52.2, 36.1–46.1 ( Stier 2012). Tail lengths (cm) for males and females, respectively, were: 22.5–30.0 and 19.0–26.0 ( Stubbe 1993), 21.6–30.0 and 21.5– 28.0 ( Ansorge 1988), 20.0–24.0 and 19.0–23.5 ( Danilov and Tumanov 1976), 18.4–24.5 and 17.6–25.0 ( Polezhaev 1998), and 19.1–28.2 and 20.8–26.9 ( Stier 2012). Ear lengths (cm) for males and females, respectively, were: 3.7–5.2 and 4.0–4.8 ( Stubbe 1993), 3.0–5.0 and 3.0–4.3 ( Ansorge 1988), 4.5–4.7 and 4.3–4.6 ( Pavlinin 1959), and 3.0–5.4 and 3.0–4.7 ( Stier 2012). Height at the withers is about 15 cm ( Lemke 1981).
Skull measurements of adult M. martes (mm; mean ± SE; males and females, respectively) were: Balearic Islands (21 males and 17 females — Lopez-Martin et al. 2006), condylobasal length (CBL) 87.8 ± 0.31 and 79.9 ± 0.24, mastoid width (MW) 36.40 ± 0.37 and 35.60 ± 0.29; Denmark (23 males and 10 females —Reig 1989), CBL 87.1 ± 0.31 and 79.2 ± 0.51, MW 42.1 ± 0.21 and 37.5 ± 0.63; Finland (37 males and 27 females — Lansink et al. 2019), CBL 85.61 ± 0.41 and 78.90 ± 0.41, MW 39.75 ± 0.24 and 36.25 ± 0.21; Karelia (72 males and 30 females — Danilov and Tumanov 1976), CBL 83.0 ± 0.24 and 76.8 ± 0.26; eastern Germany (38 males and 15 females — Monakhov and Hamilton 2020), CBL 86.41 ± 0.30 and 79.83 ± 0.30, MW 41.48 ± 0.17 and 38.36 ± 0.24; Caucasus (33 males and 34 females — Monakhov and Hamilton 2020), CBL 85.36 ± 0.32 and 78.37 ± 0.25, MW 39.53 ± 0.16 and 36.56 ± 0.13, and Kirov Oblast, Russia (32 males and 31 females — Monakhov and Monakhova 2014), CBL 80.80 ± 0.30 and 73.58 ± 0.27, MW 34.67 ± 0.13 and 33.04 ± 0.15. Thus, M. martes with the largest sizes live in the west and those with the smallest sizes in the east in Europe (Reig 1989; Monakhov 2021).
Little attention has been given to the size and structure of the baculum of M. martes ( Fig. 3 View Fig ). Stubbe (1993) provided data on the os penis (minimum–average–maximum): length, excluding age, was 38.9–42.3– 45.7 mm and mass was 0.20–0.29– 0.35 g. Vercillo and Ragni (2011) reported that baculum length of adult M. martes in Italy was 42–51 mm, and they, like Abelentsev (1968) and Pavlinin (1963), argued that this trait can be used to distinguish M. martes and M. foina . Length of baculum for M. martes of western Ukraine was 37–44.6 mm ( Abelentsev 1968) and in the upper reaches of the Pechora River basin, was 37.0–41.26–45.0 mm (minimum–average–maximum— Polezhaev 1998). Size of the baculum depends on age ( Jurgenson 1947). Dimensions (mm; minimum–average– maximum) of the os penis for juveniles were 35.1–37.0–38.3 and adults 38.9–42.3–45.7 ( Novikov 1956). Ranges reported for baculum were: 32.4–36.1–41.0 mm for 64 juvenile males and 38.9– 42.8– 46.4 mm for 67 adults from the Urals (measurements taken by VM). Some researchers consider it possible to estimate age (juveniles versus adults) based on characteristics of the baculum ( Popov 1943; Walton 1968; Malecha et al. 2009).
Pelage of M. martes is monotonous from light brown to chestnut with a yellow, rarely almost white or creamy, oblong throat patch. Tail is fluffy and dense more than one-half the body length and darker at the tip ( Stubbe 1993). Fur is practically devoid of the gray hairs and gloss that are characteristic of M. zibellina . The difference in coloration of the body and head is almost negligible ( Pavlinin 1963). Summer fur is darker than winter fur. Martes martes with albino ( Fig. 4 View Fig ), melanistic ( Fig. 5 View Fig ), or anomalously colored pelts are rarely captured ( Stubbe 1993). Pavlinin (1965) described the case of catching an individual with black skin in the south of the Perm region. Voipio (1962) believed that variation in the general color of fur and throat patch happened independently and irrespective of age. He identified three color variations in M. martes , the frequencies of which were “light” 9.5%, “medium” 50.8%, and “dark” 39.7% (n = 398). There were statistically more females with a dark pelt (49.5%) than males (30.2%— Voipio 1962). Davletov (2013) reported that there are four color categories for M. martes skins used in international trade: “dark blue,” “blue,” “dark sand,” and “sand.”
Color and shape of the throat patch (bib) of M. martes has been studied more extensively than coat color. The throat patch ( Fig. 1 View Fig ) does not extend to front feet ( Grimmberger 2014). According to Grakov (1969), only 2.6% of 5,624 pelts lacked a throat patch, and only 1.8% of the patches were white. Throat patches are yellow, orange, or white. For example, Cavazza (1912) stated that M. martes of Sardinia was characterized by a yellow-orange patch, whereas individuals in the Alps and the northern part of the peninsula had a lightyellow patch. According to Grakov (1974), the ratio of groups of skins with one of the three variants of throat patches in some populations of M. martes in Russian was (% yellow, orange, and white, respectively, ± SE): Novgorod region, 25.8 ± 3.9, 54.7 ± 4.4, and 19.5 ± 3.5; Arkhangelsk region, 78.2 ± 2.2, 18.4 ± 1.9, and 2.7 ± 1.5; Pechora River, 59.1 ± 4.4, 26.1 ± 4.7, and 2.2 ± 3.7 (no spots 12.7 ± 3.5); Kirov region, 43.0 ± 4.0, 57.0 ± 4.0, and 0.0; Bashkir Republic, 58.2 ± 3.7, 39.5 ± 3.7, and 2.3 ± 1.1; and Chelyabinsk region 9.6 ± 6.4, 90.4 ± 6.4, and 0.0.
In summer, there are 190–250 guard hairs/1,000 down hairs on the skin of M. martes ; guard hairs are 23–27 mm in length with a diameter of 100–150 μm and downy hairs are 11–12 mm and 15–16 μm, respectively ( Grakov 1978). In winter on the skin, there are 63–83 guard hairs/1,000 down hairs; and guard hairs are 36–38 mm in length with a diameter of 75–90 μm whereas the downy hairs are 22.0– 22.5 mm long and 14.0–14.5 μm ( Grakov 1978). Pavlinin (1965) obtained the following average values of hair size in M. martes (10 males and 11 females) of the Lower Ob: length of guard hairs was 40.7 and 43.1 mm, downy hairs were 23.7 and 23.8 mm in length; guard hairs had diameters of 101.0 and 101.6 μm and downy hairs 17.1 and 17.1 μm. Molting lasts from late April to early July in spring and summer and August to November in autumn. Hunters typically harvest M. martes in November– February when it has the most valuable fur. Molt in spring occurs first on the head and gradually extends to the tail; it is reversed in autumn ( Stubbe 1993).
Color of the fur on the head is almost always the same as that on the back. Tip of the nose is dark or black ( Lemke 1981; Görner and Hackethal 1988). Dense vibrissae occur on upper lips. Ears have a pale-yellow border.
DISTRIBUTION
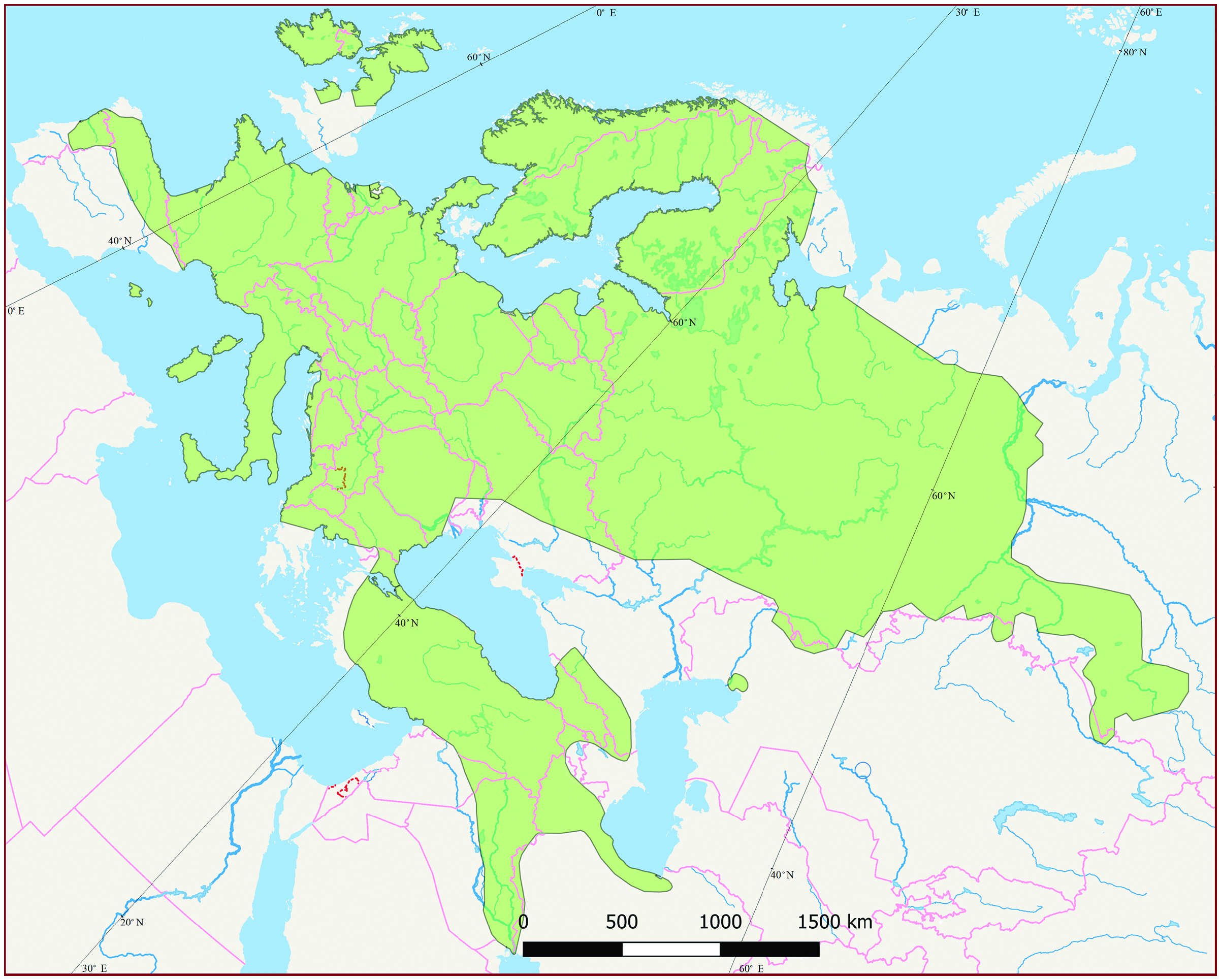
Martes martes inhabits mature mixed and coniferous forests of the temperate zone of central and western Eurasia ( Anderson 1970) from the Atlantic Ocean to the foothills of Altai Mountains ( Fig. 6 View Fig ). The area occupied by M. martes covers about 11 million km 2 of the western Palearctic: almost all of Europe, the Caucasus, Asia Minor, Iran, and a part of Western Siberia. The Russian part of its distribution area is 5.05 million km 2 (46%). Overall, the distribution is 6,800 km from west to east and 4,600 km from south to north.
Martes martes is native in Albania (east), Armenia, Austria, Azerbaijan (north), Belarus, Belgium (south), Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Georgia, Germany, Greece (north), Hungary, Iran, Ireland, Italy (with islands), Kazakhstan, Latvia, Liechtenstein, Lithuania, Luxembourg, Moldova, Montenegro, the Netherlands, North Macedonia, Norway (central and south), Poland, Portugal (north), Romania, Russian Federation, Serbia, Slovakia, Slovenia, Spain (north; Baleares —introduced from continent), Sweden, Switzerland, Turkey, Ukraine, and United Kingdom (north, west— Proulx et al. 2004; Herrero et al. 2016).
Martes martes is autochthonous throughout its distribution (for exceptions see “Conservation”). Distribution of M. martes is wide and generally continuous, without significant isolated areas. The latter include (except for islands) the population in Wales and that in the lower reaches of the Ural River (Atyrau region, Kazakhstan). Martes martes occurs at elevations up to about 2,300 m in the Pyrenees Mountains and Spain ( Herrero et al. 2016), and at 2,200 –2,400 m in the Caucasus ( Bakeev 1973).
FOSSIL RECORD
The genus Martes is poorly differentiated among mustelids and dates to the Miocene and probably from the late Pleistocene of central and western Europe. After the Last Glacial Maximum, it entered Scandinavia ( Stubbe 1993; Sommer and Benecke 2004). Anderson (1970, 1994) considered M. laevidens Dehm, 1950 from lower Miocene deposits in Bavaria, Germany, to be the earliest known marten ancestor, and the line of phylogeny imagined as Martes laevidens – M. wenzensis – M. vetus (intermedia M. foina )– M. martes . It was a small marten with thin jaws and four premolars in each side of the jaw. Later, near Lodz, Poland, remains were found of a similar but larger M. wenzensis Stach, 1959 , from the early Villafranchian horizon (3.5–1.0 Ma), and a similar Martes from Greece and Romania ( Anderson 1994). Nevertheless, it has recently been found that M. laevidens does not belong to the true marten group based on anatomical features, and M. wenzensis should be considered the ancestor of true martens ( Sato et al. 2003). In the middle-to-late Pleistocene, M. martes and M. zibellina became distinct species ( Anderson 1994). Ancestors of M. martes and M. zibellina ( M. vetus ), morphologically like modern species, are known from several Riss- Wurm deposits from the Pleistocene in central Europe ( England [Kent and Devon], Austria, Germany [Westphalia and Weimar], Czech Republic, Poland, Hungary, and the Netherlands) and Holocene from Norway, Denmark, Switzerland, Belgium, Bulgaria, France, Italy, and Romania ( Anderson 1970). Sato et al. (2003) estimated divergence of M. martes and M. zibellina at 0.43 Ma, but others estimated this separation at about 2 Ma ( Bininda-Emonds et al. 1999; Hosoda et al. 2000).
The earliest unquestioned find of M. martes belongs to the last interglacial period, the Eemian ( Wolsan 1993; Anderson 1994; Van Kolfschoten 2000), about 120,000 years ago. Fossils that might represent M. martes have also been found in deposits extending as far back as 400,000 years ago ( Wolsan 1993). Sommer and Benecke (2004) published a study on glacial refugia in southern Europe, summarizing fossil findings of mammals, including M. martes . For the late glacial, there are 20 M. martes localities with the northernmost record in Denmark. The Iberian and Italian peninsulas and Carpathians ( Moldova) are considered glacial refuges for M. martes , and the Balkans and North Pontic region as possible refugia ( Sommer and Benecke 2004).
Fossil remains of M. martes are also known from the layers of the middle-to-upper Pliocene near Odessa in Ukraine ( Gromova 1962), on the territory of Moldova ( Svistun et al. 1989), near Pavlodar, Kazakhstan, the Caucasus, and Crimea ( Gromova 1962; Baryshnikov et al. 1981). Numerous Holocene samples have been described from localities near the cities of Vinnitsa, Kiev, Poltava, Khmelnitsky, Chernivtsi, and Chernigov ( Abelentsev 1968). In the Southern Urals (near Magnitogorsk and Ufa), M. martes was recorded in Pleistocene horizons in mountainous areas with ages of 11,000 –35,000 years BC and throughout the Holocene in mountains and on the plains ( Kosintsev and Bachura 2013). Martes martes entered the Central Urals in the middle Holocene and into the Northern Urals in the late Holocene ( Kosintsev et al. 2016). Based on samples from the Subboreal period (2,600 –4,000 years BC), M. martes is thought to have spread to the right bank of the Yenisei, and its distribution moved west near the right bank of the Ob in the modern Tomsk region ( Devjashin et al. 2016).
Huges (2012) drew an updated scheme of the phylogeny of Martes that added new fossil finds related to older ancestors. For example, she included species such as M. munki , M. filholi , M. burdigaliensis , M. collongensis , M. sainjoni , and others with age estimates of 15–18 Ma from France, Spain, and Austria. It confirmed Anderson’s (1970, 1994) scheme for the ancestors of true martens of the Miocene ( M. wenzensis ) and the Pleistocene ( M. vetus ), although their relationship was disputed by Kurten (1968), and general conformity with new genetic data ( Huges 2012). A new fossil species M. crassidens from the early-tomiddle early Pleistocene horizon from the vicinity of the city of Dalian ( China) was recently described by Jiangzuo et al. (2021) who concluded that this marten was closely similar (even more than wenzensis and vetus ) to the martes – zibellina – melampus – americana group (Holarctic marten group). They believed that they found a true ancestor (including M. martes ) of the Holarctic marten group that originated in this region of Asia and spread almost throughout the Holarctic ( Jiangzuo et al. 2021).
Many mammals of Eurasia are representatives of migratory faunas at different periods of their species history, and Anderson (1970) referred the genus Martes to the same category. During the long phylogenesis of M. martes , alternate periods of increase and decrease in body size occurred ( Paaver 1965; Smirnov 1975). By analyzing (radiocarbon dating) subfossil samples, the time of translocation of M. martes to the island of Mallorca was proposed. Valenzuela and Alcover (2015) examined two samples and determined a date range of 87–330 AD (i.e., translocations took place during the Roman Empire).
FORM AND FUNCTION
Form. —Structural features and size of the skull can be used to determine age of M. martes ( Ansorge 1988, 1992; Reig and Ruprecht 1989; Helldin 1997, 1999). Tooth wear, developing masticatory muscles of the skull, development of the tibia epiphysis, size of the baculum, and other features that have been described for the fisher ( Pekania pennanti ) and M. americana ( Marshall 1951; Poole et al. 1994), and have been used to separate young individuals from older ones. To estimate age of adult M. martes, Helldin (1997 , 1999) counted annual layers in cementum of canine teeth as described for the fisher ( Strickland et al. 1982). Similar methods to estimate the age of M. zibellina and M. martes were used earlier in the Soviet Union ( Stroganov 1937; Timofeev and Nadeev 1955; Jurgenson 1956; Maldzhiunaite 1957; Smirnov 1960; Ryabov 1962; Grakov 1963; Klevezal and Kleinenberg 1967; Pavlinov 1976).
Dentition of M. martes is very similar to that of M. zibellina and M. americana (Clark at al. 1987). Dental formula is i 3/3, c 1/1, p 4/4, m 1/2, total 38. Length of upper toothrow (mm; 47 males and 30 females, mean ± SE) in M. martes from eastern Germany was 30.5 ± 0.19 and 28.6 ± 0.18, and length of lower toothrow was 35.1 ± 0.18 and 32.8 ± 0.20 ( Ansorge 1988). Mean length of upper molar (mm; 29 males and 20 females) was 6.5 and 5.8, and its width was 8.6 and 7.9 in Sweden ( Stubbe 1993). Length of the lower molar in M. martes from Poland (mm; mean ± SE; 133 males and 103 females) was 9.9 ± 0.32 and 9.0 ± 0.34 (Reig 1989). Wolsan (1989) gave a classification of morphotypes according to shape and size of teeth for species of Martes that was later used to identify M. zibellina , M. martes , and M. foina (Kosintsev and Gimranov 2015) .
Vertebral formula of M. martes is 7 C, 14 T, 6 L, 3 S, 16–19 Ca, total 46–49 ( Stubbe 1993), but Heptner et al. (1967) reported the number of caudal vertebrae as 15–22. Partial spinal lengths (mean % of the total length of the spine and parenthetical range) were: cervical 20.2 (19.6–20.7), thoracic 43.7 (43.0–44.2), lumbar 29.3 (29.0–29.8), sacral 6.8 (6.5–7.1), and tail 73.5 (70.8–77.9— Stubbe 1993). Female M. martes have two ( Görner and Hackethal 1988) or three pairs ( Grakov 1981; Stubbe 1993) of teats. Lactation lasts about 2 months ( Starkov 1940).
The heart of M. martes is reniform, located between the 6th and 10th rib, and its apex is directed caudolaterally. Heart index (heart-to-body mass ratio; ‰ ± SE) of 49 males and 38 females, respectively, was 9.3 ± 0.2 and 9.5 ± 0.2 ( Tumanov 2003) and of 53 males and 38 females was 8.66 ± 0.22 and 8.91 ± 0.29, respectively ( Polezhaev 1998). Diameter of the aorta was 4.8 mm near the heart and 2.5 mm at the trifurcation; length of aorta was 36.5% of the body length ( Tumanov 2003). The thoracic part of aorta was 52.3% of its total length. Diameter of the vena cava was 5.4 mm, external jugular 3.5 mm, internal jugular 2.0 mm, subclavian 3.3 mm, renal 3.5 mm, and portal 3.3 mm ( Tumanov 2003). Kidney index (kidney-to-body mass ratio, ‰ ± SE) was 3.7 ± 0.17 and 4.25 ± 0.32 for 53 males and 38 females, respectively; lung index (lung-to-body mass ratio, ‰ ± SE) was 13.15 ± 0.79 and 14.51 ± 0.97 in 53 males and 38 females, respectively; spleen index (spleen-to-body mass ratio, ‰ ± SE) for 53 males and 33 females was 2.43 ± 0.13 and 2.19 ± 0.24; and adrenal gland index (adrenal-to-body mass ratio, ‰ ± SE) for 29 males and 27 females was 0.05 ± 0.023 and 0.06 ± 0.016 ( Polezhaev 1998).
Intestinal lengths of 33 male and 19 female M. martes (% ± SE) relative to body length was 436 ± 8.5 and 405 ± 8.2 for small intestines and 38 ± 1 and 39 ± 1.4 for large intestines; liver-to-body mass ratio (% ± SE) in 48 males and 37 females was 3.4 ± 0.6 and 3.5 ± 0.7 ( Tumanov 2003) and in another 53 males and 38 females 3.51 ± 0.18 and 3.78 ± 0.19 ( Polezhaev 1998). The stomach of M. martes can contain up to 60–90 g of food, but most often it contains up to 50 g ( Heptner et al. 1967), with a maximum of 115 g and an average mass of 35.5 g ( Danilov and Tumanov 1976).
Function. —Body mass of Martes martes changes seasonally. In northwestern Russia, maximum female body mass of 1 kg occurred in September and near maximum mass of 0.95 kg occurred in May and October–November and 0.92 kg in April and December; minimum body mass of 0.85 kg occurred in June ( Tumanov 2003). These changes were consistent with seasonal dynamics in daily food consumption (g) for five males (average body mass 1.22 kg) and five females (average body mass 0.89 kg), respectively: 243 g and 191 g in spring, 212 g and 182 g in summer, 295 g and 220 g in autumn, and 232 g and 208 g in winter, or an average of 26.6 and 29.7 kcal/ 100 g body mass. Daily caloric need in kcal for an individual weighing 0.93 kg was 268.1 in spring, 264.4 in summer, 335.3 in autumn, and 271.7 in winter, or an average of 30.6 kcal/ 100 g of body mass ( Tumanov 2003). Seasonal dynamics of body mass and the need for food determine the physiological state of an individual and its reproductive condition. Seasonal dynamics of adrenal gland mass of 27 females in autumn–winter (mean ± SE) and five females in spring–summer were 85.6 ± 4.1 mg and 96.3 ± 3.5 mg, and the thickness of the cortical layer was 0.90 ± 0.05 mm and 0.95 ± 0.06 mm, respectively ( Tumanov 1993). Martes martes has a rather high cerebral index (brainto-body mass ratio, ‰ ± SE; n = 12) of 18.4 ± 0.8, higher than that of the Eurasian polecat ( Mustela putorius ), American mink ( Neovison vison , currently Neogale vison ), European mink ( Mustela lutreola ), Eurasian otter ( Lutra lutra ), wolverine ( Gulo gulo ), and European badger ( Meles meles — Tumanov 2003).
Martes martes molts in spring and autumn; onset of molt is associated with photoperiod. Spring molting begins in March and lasts through early June, and autumn molt is from late August to early November. Timing of molting depends on condition and nutrition of the individual, its age, and weather; molt occurs faster in autumn ( Ternovsky 1977). In northern parts of the distribution, spring molting begins 1–2 weeks later than southern parts ( Danilov and Tumanov 1976). Martes martes has its complete winter fur for about 5 months and its summer fur for about 2 months. Adult females molt faster than males, and less well-fed individuals molt faster than fat or sick individuals ( Starkov 1947).
Average monthly body temperatures of captive female M. martes were 37.6–39.5°C, being lowest in August and October and highest in March ( Tumanov 2003). Respiration rate of 15 captive adult males was 59/min; pulse rate was 325 ± 8.0 for eight males and 345 ± 17.8 for three females ( Tumanov 2003). Heart rate was 259 beats/min/kg of body mass for males and 312 for females; breathing frequency was 27 breaths/min for males and 31 for females ( Ternovsky et al. 1981).
Water and energy metabolism of M. martes was compared with the steppe polecat ( Mustela eversmanii ) in 1995 and M. zibellina in 1998 at Chernogolovka Research Station of Moscow province ( Meshcherskii et al. 2003). A high level of metabolism was noted in M. martes and M. zibellina , as expressed in a greater amount of digestible food (coefficient of dry food digestibility, 92.5 ± 0.5 SE), water (total water intake, 216 ± 34 g /kg of body mass/day), and correspondingly higher evaporative water loss (proportion of evaporative water losses in the total balance, 49.1 ± 4.3%) and heat dissipation (proportion of energy taken in that was dissipated with evaporation, 28.0 ± 2.4%), compared with the steppe polecat ( Meshcherskii et al. 2003).
ONTOGENY AND REPRODUCTION
Ontogeny. —At birth, Martes martes weighs 20–27 g (Ternovsky and Ternovskaja 1994), its auditory canals are closed (open at 28–33 days), and it is blind (eyes open at 30–36 days). Young are covered with light gray juvenile down that becomes gray-brown fur in a few days. At 40–45 days of age, young develop canines and incisors. Juveniles feed on mother’s milk for 1.5–2 months and then gradually switch to meat ( Aspisov et al. 1967; Grakov 1981). Young leave the nest at 37–40 days old. By July at the age of 10 weeks, juveniles weigh about 0.4 kg. Rather rapid growth of juveniles continues until autumn when they reach 0.6–0.7 kg (80% of adult body mass), and they are hardly distinguishable from their parents in appearance. During this period, families break up, and juveniles transition to an independent life ( Danilov and Tumanov 1976). Young M. martes molt in September ( Heptner et al. 1967).
Based on CBL and mass of mandible Pavlinov (1977) showed that the skull of M. martes grows slowly during almost the animal’s entire life, but a higher specific growth rate was recorded in the period from 10 months to 1+ years of age; a noticeable decrease in specific growth rate was recorded from 4 to 7 years of age. Skull sizes in four age groups of M. martes were mean CBL (mm, males and females, respectively) of 81.0 and 74.7 for juveniles, 82.2 and 75.7 for yearlings, 83.2 and 76.3 for 2+ years old, and 81.8 and 75.5 mm for 3+ years old and mean mastoid width (mm) of 35.6 and 34.3 for juveniles, 35.6 and 34.2 for yearlings, 35.0 and 32.6 for 2+ years old, and 34.8 and 32.9 mm for 3+ years old ( Polezhaev 1998). The growth rate of the skull of M. martes on average begins to decrease after 2 years of age ( Yazan 1972).
In the wild, M. martes rarely lives up to 10 years; the proportion of individuals reaching 6–7 years of age was 5.1%, 7–8 years was 3.6%, and 10+ years of age was 1.4% (n = 646— Pavlinov 1977). A hunting sample of 1,245 individuals contained 1.5% 5-year-olds and only 2.5% 6-year-olds and above; maximum age of males was 11–16 years ( Grakov 1981). Siivonen (1979) noted that life expectancy of M. martes was 8–10 years and rarely up to 15 years. In captivity, M. martes lives up to 18 years ( Starkov 1940).
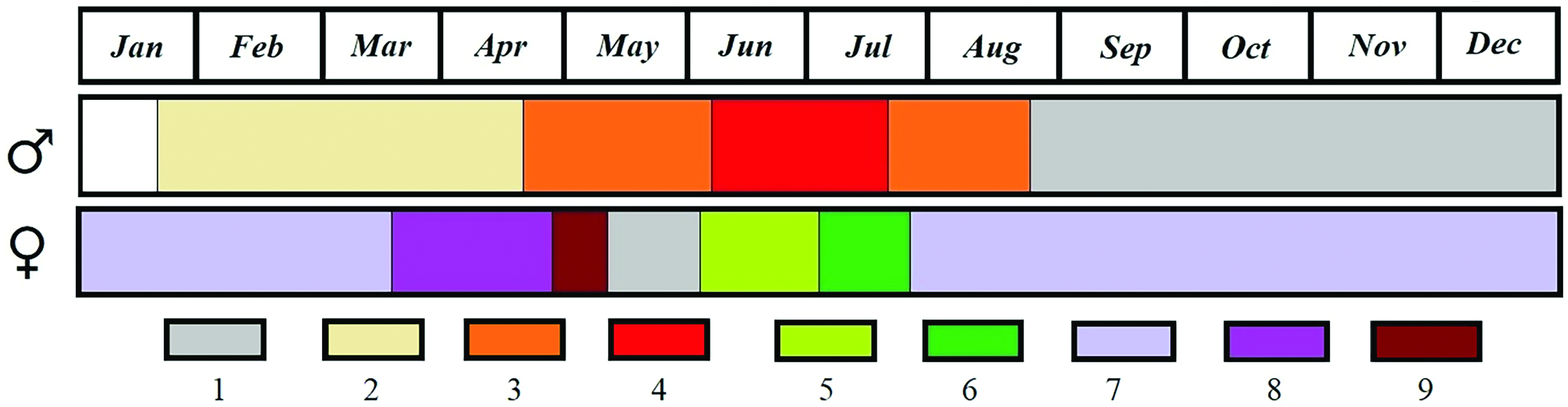
Reproduction. —Breeding season of Martes martes is from late June to mid-August ( Stubbe 1993) and usually occurs in July–August ( Aspisov et al. 1967). Danilov and Tumanov (1976) provided detailed data on the sexual cycle of male and female M. martes and indicated that estrus in females was rather short, from late June to late July, but active spermatogenesis in males was much longer, from late April to late August ( Fig. 7 View Fig ). Ovulation was triggered by mating ( Kler 1941).
Schmidt (1943) indicated a gestation period of 249–286 days (n = 19) with birth dates from 10 March to 5 May. Ternovsky and Ternovskaya (1994) reported gestation at 265–279 days (n = 5), and Tumanov (2003) reported 240–264 days (n = 10). At an experimental cage farm, birth of young was recorded only in the morning ( Tumanov 2003). Births in central Germany occurred in March and April ( Stier 2012). Mating dates on the experimental farm study by Ternovsky and Ternovskaya (1994) were from 6–12 July to 25 July, with coitus lasting up to 150 min (average 50 min— Schmidt 1943). In the wild, mating occurred 14 June–30 July ( Germany — Stier 2012). During the second year of life, 10–45% of females become reproductive ( Heptner et al. 1967; Danilov and Tumanov 1976; Stubbe 1993). Up to 10% of adult females 2+ years old do not reproduce ( Grakov 1981). In captivity, females generally enter estrus at 3 years of age ( Grakov 1981; Stubbe 1993), although a tame female at 456 days of age mated with a wild male and gave birth after 264–274 days ( Austria — Krott 1973). The peculiarity of the structure of the female reproductive system, with the ovary inside the oviduct, excludes ectopic pregnancies in M. martes ( Manteifel 1934) .
The first reported M. martes offspring born in captivity was at the Moscow Zoo ( Manteifel 1934). Based on daily observations that began in 1927 at the zoo, dates of mating for M. martes were 28 and 29 July 1928, and the first litter (a single offspring) was born after 237 days on 22 March 1929. This female also mated on 18–22 July 1929 and 21–25 July 1930 and had two young on 24 March 1930 and four young on 26 March 1931, respectively ( Manteifel 1934). These observations showed that the breeding biology of M. martes was very similar to M. zibellina , suggesting hybridization could potentially occur.
In the 1920s and 1930s, breeders in Europe and North America were unsure about the regular breeding patterns of four species of Martes : americana , martes , pennanti (currently considered Pekania pennanti ), and zibellina ( Ashbrook and Hanson 1927, 1930). A common mistake of breeders, taken from hunters, was that the mating season of Martes took place in February–March; however, in the 1920s, researchers began to doubt this. Numerov (1969) confirmed that summer breeding of captive males and females resulted in the first offspring the following spring: M. martes on 22 March 1929 at the biological station of the Moscow Zoo, M. zibellina (ibid.) on 3 April 1929, M. foina on 7 April 1929 at Hirschegg-Riezlern farm, Germany ( Austria at present), and M. americana on 23 April 1929 on a farm in Saratogа Springs, New York, United States ( Ashbrook and Hanson 1930).
Dimensions of the genitals of young and adult M. martes differ markedly. In juvenile males, mass of testes with appendages in winter was 120–260 mg (mean, 210), and in adults, 210–520 mg (mean, 350); in June–July, mass of the genitals of adult males reached 1,900 –2,500 mg (northwestern Russia — Danilov and Tumanov 1976). Stier (2012) proposed that sperm in the testes of M. martes in Germany was present only when the genitals weighed more than 1,300 mg. Spermatozoa of M. martes and M. zibellina are the same in size and shape. Length of the sperm head is about 8 µm, width is about 6 µm, and length of the tail is about 55 µm ( Starkov 1947).
Mean mass of the uterus of juvenile females was 82 mg in autumn and can be as large as 205 mg in winter, 375 mg in a pregnant female, and 865 mg in March. Mean mass of the uterus during a female’s first pregnancy in winter is 308 mg compared to 402 mg in a female with repeated pregnancies ( Gribova 1956). In January–February, genital mass was 280–430 mg (on average 330) in juvenile females and 680–1,280 mg (on average 940) in adult females ( Danilov and Tumanov 1976). Size of the embryo blastocyst during the latent stage (6–7 months) does not change much and is 0.8–1.0 mm ( Starkov 1947). At the end of winter (before implantation), sizes of the corpora lutea in ovaries are about 1,210 –1,349 μm ( Gribova 1956) but by March increase to 1,700 –1,800 μm ( Danilov and Tumanov 1976). Based on the analysis of the number of corpora lutea of pregnancy (CLP) in the ovaries, Grakov (1981) found that the proportion of pregnant females at 1 year of age was 82%, 2 years 90%, 3 years 100%, 4 years 92%, 5 years 100%, and 6 years 100%. Those same females had an average of 3.0, 3.42, 3.25, 3.46, 3.34, 4.0, and 3.28 CLP in the ovaries, respectively ( Grakov 1981). Thus, highest reproduction in the wild is characteristic of females 3–5 years old.
The most reliable data on the number of young in a litter come from captive M. martes ; 1–4 young/litter, on average 3.0 ( Schmidt 1943) but one litter of eight young was recorded on a farm in Pushkino, Moscow region ( Portnova 1941). According to data from a survey of Russian hunters in 1959–1964, an average of 3.55 young/litter (range 1–8) in 1,184 nests, a maximum of 4.9 young in 34 litters in the Kaliningrad region, and a minimum 3.38 young in 314 litters in northern regions of Russia ( Grakov 1981). After giving birth, females remain in the nest for the first 2 days, then leave their litters for 5–10% of the day during the rest of the first week, 30% of the day from the second week to 1 month, and 40% of the time after that ( Stier 2012). Mortality of young while in the nest is estimated at 24–25% ( Danilov and Tumanov 1976).
Number of young (mean ± SE) in wild litters was 3.8 ± 0.35 in the Arkhangelsk region (n = 49 litters), 3.3 ± 0.09 in Vologda (n = 186), 3.2 ± 0.14 in Kirov (n = 49), 3.8 ± 0.15 in Sverdlovsk (n = 48), and 3.5 ± 0.13 in Perm (n = 57— Grakov 1981). To estimate population growth, fecundity of females can also be assessed by counting the number of corpora lutea in ovaries of females caught by hunters. The number of CLP (mean ± SE) in pregnant females was 4.13 ± 0.28 in the Arkhangelsk region (n = 19), 3.24 ± 0.19 in Vologda (n = 33), 3.60 ± 0.14 in Kirov (n = 42), 3.38 ± 0.14 in Sverdlovsk (n = 36), and 3.24 ± 0.19 in Perm (n = 18— Grakov 1993).
For about 50 years, management of hunting of M. martes in Russia has used the phenomenon of delayed implantation (and analysis of the age–sex structure of hunting samples) to predict population growth ( Grakov 1993). Female M. martes harvested in winter contained CLP in their ovaries, which were detected histologically and counted. Based on such calculations, it is possible to estimate number of offspring in the next spring and plan the hunting quota for the next hunting season. With systematic monitoring, embryonic mortality can be disregarded, and the number of CLP in a female’s ovary is equated to the number of young in a litter ( Grakov 1981, 1993).
Many researchers described the phenomenon of “spring excitement” or pseudomating in species of Martes ( Kler 1941; Grakov 1964, 1973). Such arousal is characteristic of adults and juveniles that have not yet reproduced. It is not directly related to implantation of embryos at the end of winter and not recorded in every spring. Tumanov (2003) believed that pseudomating was an ancestral feature in the behavior of M. martes , acquired by species of Martes because of glacial changes in the climate, but Grakov (1964) considered this to occur in the beginning of the formation of pair bonds between males and females.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Martes martes ( Linnaeus, 1758 )
| Monakhov, Vladimir 2022 |
Martes martes kuznetsovi
| Pavlinov I. Ya. & Rossolimo O. L. 1987: 61 |
Martes martes minoricensis Alcover, Delibes, Gosálbez, and Nadal, 1986:331
| Alcover J. A. & Delibes M. & Gosalbez J. & Nadal J. 1986: 331 |
Martes martes sabaneevi
| Jurgenson P. B. 1947: 147 |
Martes martes lorenzi
| Ognev S. I. 1926: 47 |
Martes martes ruthena
| Ognev S. I. 1926: 49 |
Martes martes latinorum
| Barrett-Hamilton G. E. H. 1904: 389 |
Martes abietum
| Gray J. E. 1865: 104 |
Martes sylvatica
| Nilsson S. 1847: 41 |
Martes vulgaris
| Griffith E. 1827: 123 |
Mustela sylvestris
| Oken L. 1816: 1029 |