Konobelodon robustus, Wang & Shi & He & Chen & Yang, 2016
|
publication ID |
https://doi.org/ 10.5252/g2016n1a4 |
|
publication LSID |
urn:lsid:zoobank.org:pub:AD9C6BAE-8A37-4201-BFA6-C49739CFDD60 |
|
persistent identifier |
https://treatment.plazi.org/id/1858A9DB-FA28-4067-8AF1-B90BB9B6316A |
|
taxon LSID |
lsid:zoobank.org:act:1858A9DB-FA28-4067-8AF1-B90BB9B6316A |
|
treatment provided by |
Felipe |
|
scientific name |
Konobelodon robustus |
| status |
sp. nov. |
Konobelodon robustus n. sp.
( Figures 3-12 View FIG View FIG View FIG View FIG View FIG View FIG View FIG View FIG View FIG View FIG ; Tables 1 -14)
Tetralophodon sp. – Deng et al. 2004: 11; 2013: 256, 257. — Deng 2006: 153.
Tetralophodon exoletus – Deng et al. 2004: 11; 2013: 257, 258.
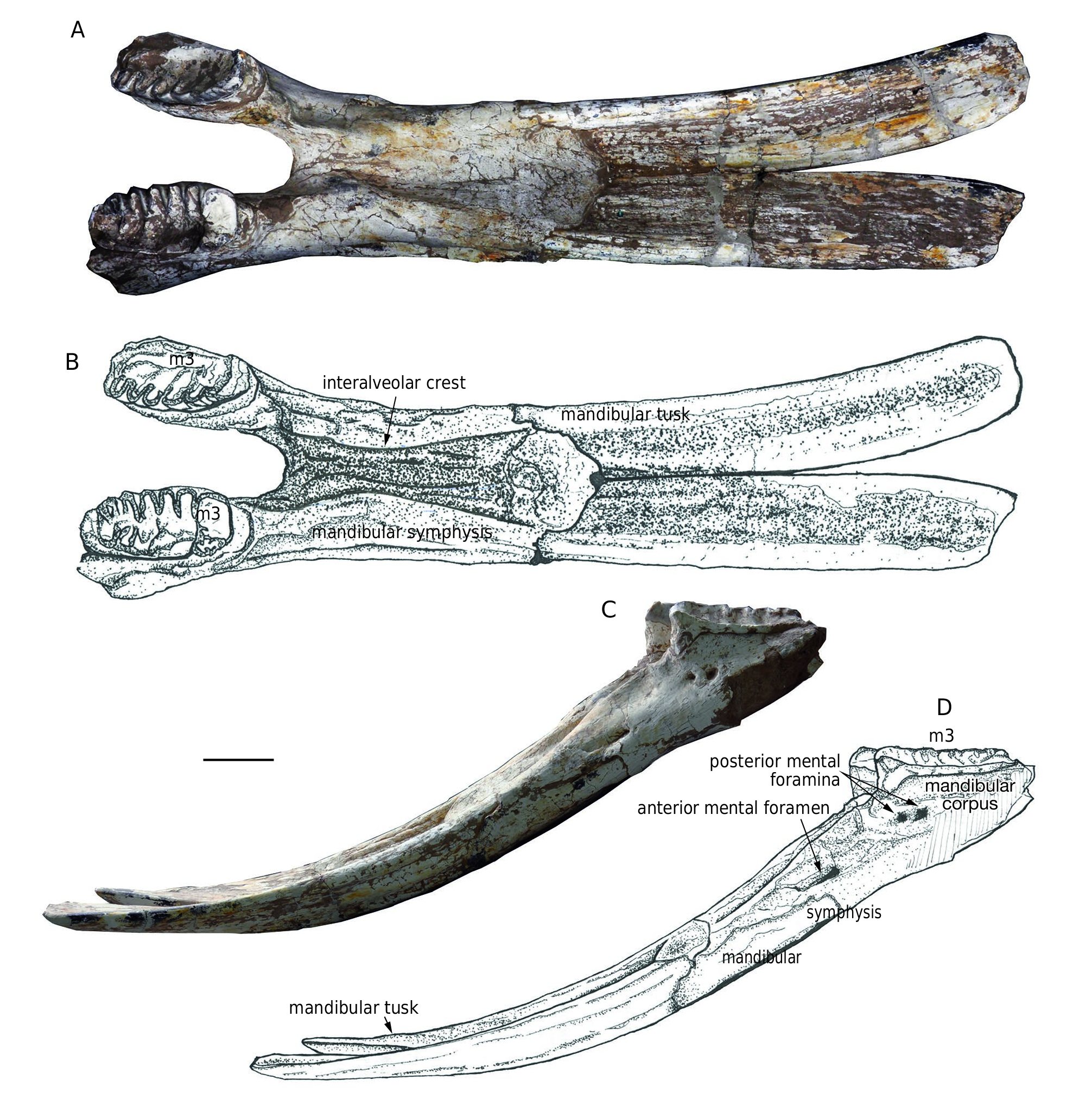
HOLOTYPE. — HMV 0004 , an incomplete adult mandible with m3s, dental age XXIII ( Fig. 3 View FIG ), loc. LX200049.
PARATYPES. — HMV 0011 , almost complete sub-adult mandible with p4 and m1, dental age XII ; HMV 1909 , almost complete juvenile mandible with both dp2s, dp3s, and dp4s, dental age V ; HMV 1910 , nearly complete juvenile cranium with both DP2s, DP3s, and DP4s, dental age IV, possibly to be the same individual as HMV 1909 , loc. of the above, LX200027 ; HMV 1904 , fragmentary cranium with both DP3s, DP4s, dental age VII, loc. LX200009 .
ETYMOLOGY. — Robustus, stout or thick, from the robust limb bones in the taxon.
REFERRED MATERIAL. — Loc. LX200049: HMV 0003, mandible with both dp3s and dp4s, dental age VII. — Loc. LX200042: HMV 1888, left dentary with dp4 and p3, dental age VIII; HMV 1889, left palate with DP2, DP3, and DP4, dental age III; HMV 1861, fragmentary left lower tusk. — Loc. LX200027: HMV 0001, mandible with both m2s, dental age XVI, however, two DP3s were incorrectly fixed on the alveoli of m1; HMV 1883, left femur; HMV 1882, left humerus; HMV 1886, left ulna; HMV 1908, mandible with both dp2s and dp3s, dental age III. — Loc. LX200009: HMV 1890, left lunar; HMV 1891, left humerus; HMV 1905, fragmentary cranium with right DP2, DP3, and partial DP 4 in alveolus, dental age III. — Loc. LX200204: HMV 1887, mandible with both m1s, however, p3s and p4s absent, dental age XIII. — Loc. LX200037: HMV 1787, fragmentary right lower tusk. — Loc. LX200007: HMV 0013 and 0002, left femurs. — Loc. LX200008: HMV 1892 and 1893, right and left dentaries with dp2 and dp3, respectively, dental age II; HMV 1894, right metacarpal IV; HMV 1895, right ulna; HMV 1896, right radius; HMV 1897, right femur; HMV 1911, left humerus; HMV 1899 and 1901, crania with both DP2s, DP3s, and partial DP4s in alveoli, dental age III; HMV 1900, mandible with left dp2, both dp3s, and partial dp4s in alveoli, dental age IV; HMV 1902, cranium with left P3, both P4s, M1s, and partial M2s in alveoli, dental age XIII; HMV 1907, mandible with both p3s, dp4s, and m1s, dental age IX; HMV 1879. 1-3, three fragmentary tusks, two are lower (1 and 2) and one is upper (3); IVPP V18970 View Materials , almost complete pelvis with partial sacrum.
Precise localities unknown: HMV 1881, atlas; HMV 1884, palate with both DP3s and DP4s, dental age IV; HMV 0539, right ulna; HMV 1906, cranium with both DP2s, DP3s, and DP4s, dental age V; HMV 1456 cranium with associated mandible with DP2s/ dp2s, DP3s/dp3s, and partial DP4s/dp4s in alveoli, dental age III; HMV 1903, mandible with both p3s, dp4s, and m1s, dental age X.
TYPE HORIZON. — Early Late Miocene, estimated as 11.1-9.8 Ma (corresponding to the European Vallesian, MN 9/ NMU 8).
STRATIGRAPHICAL AND GEOGRAPHICAL DISTRIBUTION. — MN 9- MN 10 ( NMU 8- NMU 9), northern China.
DIAGNOSIS. — Neurocranium moderately domed; basicranium moderately erected; mandible with extremely elongated symphysis but not laterally expanded in the distal part; symphysis moderately downwardly deflected and high at the base; upper tusks ventrally bent (at least in the juvenile stage) without lateral enamel band; lower tusks divergent in dorsal view and with tubular internal dentine; exposed length of lower tusks in adults longer than symphyseal length; cross-section of lower tusks dorsally concave and ventrally convex without any concave emargination; DP4/dp4, M1/m1, and M2/m2 bunolophodont and tetralophodont; m3 bunolophodont and hexalophodont; pretrite half-loph(id)s trifoliated; posttrite trefoils weak.
DIFFERENTIAL DIAGNOSIS. — Differs from Konobelodon atticus in the narrowness of the lower tusks, in the absence of a ventral groove on the lower tusks, in thinner tubular structure in the cross-section of the lower tusk, and in the more divergent lower tusks in juveniles. Differs from Konobelodon britti in the smaller size of molars and lower tusks, in the absence of an enamel band in the upper tusks, in the more incipient states of secondary trefoils and pseudo-anancoidy in the cheek teeth.Differs from Platybelodon in the relatively domed neurocranium and the relatively erected basicranium, in the not laterally expanded (in the distal part) and downwardly deflected symphysis, in the not posteriorly oblique mandibular rami, in the divergent lower tusks (in dorsal view), in the longer exposed length and the not much flattened cross-section of lower tusks, and in the tetralophodont DP4/dp4, M1/m1, and M2/m2. Differs from Amebelodon in the not laterally expanded (in the distal part) symphysis, in the absence of an enamel band in the upper tusks, in the tetralophodont DP4/dp4, M1/m1, and M2/m2, in the more incipient states of secondary trefoils and of pseudo-anancoidy in the cheek teeth, and in the presence of dentinal tubular structure in the lower tusks. Differs from Tetralophodon logirostris in flattened cross-section of lower tusks, and in the presence of dentinal tubular structure in the lower tusks.
ANATOMICAL DESCRIPTION AND COMPARISONS
Mandible ( Figs 3 View FIG ; 4 View FIG ; Table 1)
Holotype ( Fig. 3 View FIG ). HMV 0004 is an incomplete mandible missing the mandibular rami and the posterior parts of the mandibular corpuses. However, it is the only adult individual in the study material. In dorsal view, the symphysis is almost twice the length of the maximal width; however, the distal part of the symphysis is not transversely expanded. In the basal part, the two interalveolar crests are closed to each other, forming a narrow medial groove. The interalveolar crests are divergent in the distal part and rapidly reach the anterolateral symphyseal borders. The anterodorsal edge of the symphysis is anteriorly convex rather than straight. In lateral view, the symphysis is moderately downwardly deflected. The height of the symphysis at the base is large, almost equal to the height of the corpus. The posterior mental foramina, which are slightly posterior to the level of the anterior end of the tooth row, are duplicated. The anterior mental foramen is anteroventrally elongated.
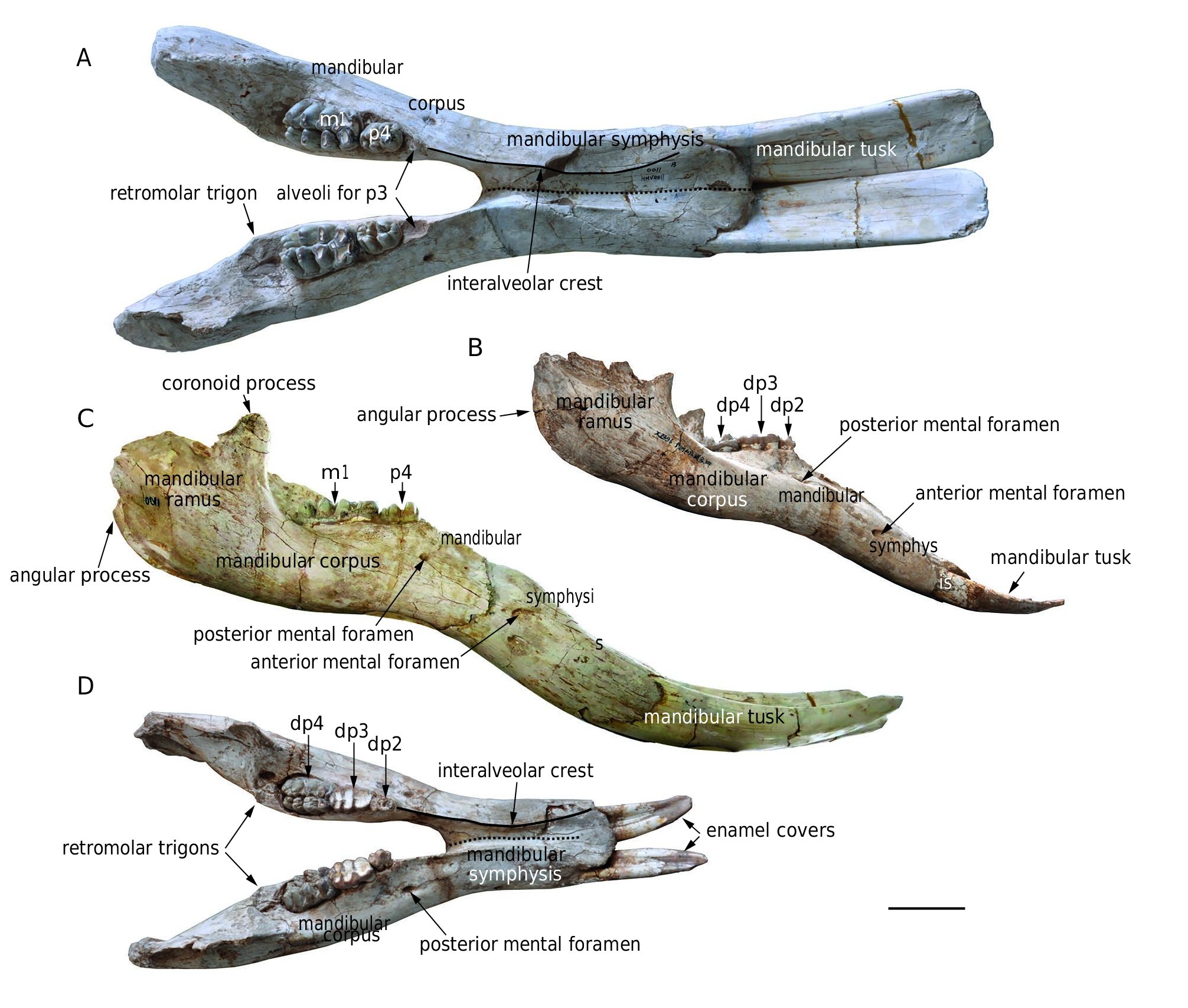
Other mandibles ( Fig. 4 View FIG ). In addition to the holotype, there are 12 mandibles ( HMV 1887, 1892, 1893, 0001, 0003,
0011, 1456, 1900, 1903, 1909, 1907, 1908) in the study material. Except for HMV 1892, 1456, and 1900, all other mandibles possess in situ lower tusks.Within the 12 mandibles, HMV 1887, 0001, 0011, 1903, and 1907 are sub-adults (at least m 1 in use), and the others are juveniles. The following description is mainly based on the paratypes HMV 0011 (a sub-adult) and HMV 1909 (a juvenile).
The mandibular ramus is long and shallow with a strong, upward-protruding coronoid process. The angular process is weakly developed, and is at the level of or slightly higher than the occlusal surface of the cheek tooth row. The anterior and posterior ramal borders are perpendicular to the occlusal surface and less posteriorly inclined than those in some taxa such as Platybelodon grangeri ( Wang et al. 2013b) and Gomphotherium angustidens ( Tassy 2013) . The corpus is strong, with a prominent retromolar trigon. The corpus tapers anteriorly in dorsal view and increases in height anteriorly in lateral view. Differing from the duplicated posterior mental foramina in the holotype, there is only one posterior mental foramen in HMV 0011 and HMV 1909. The morphology of the symphyseal part is almost the same as the holotype except that, in lateral view, the downward deflection of the symphysis is not as strong as that in the holotype.
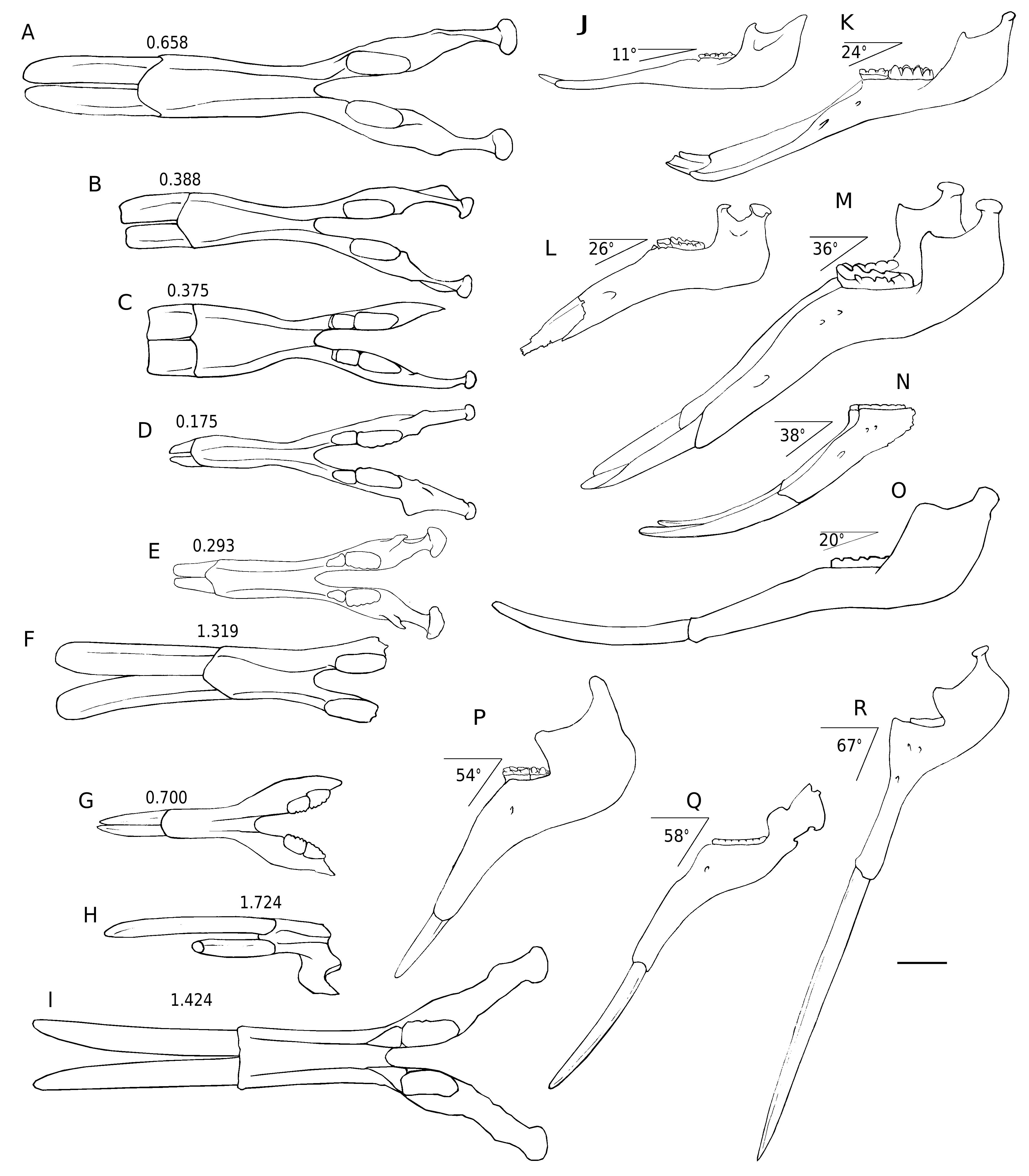
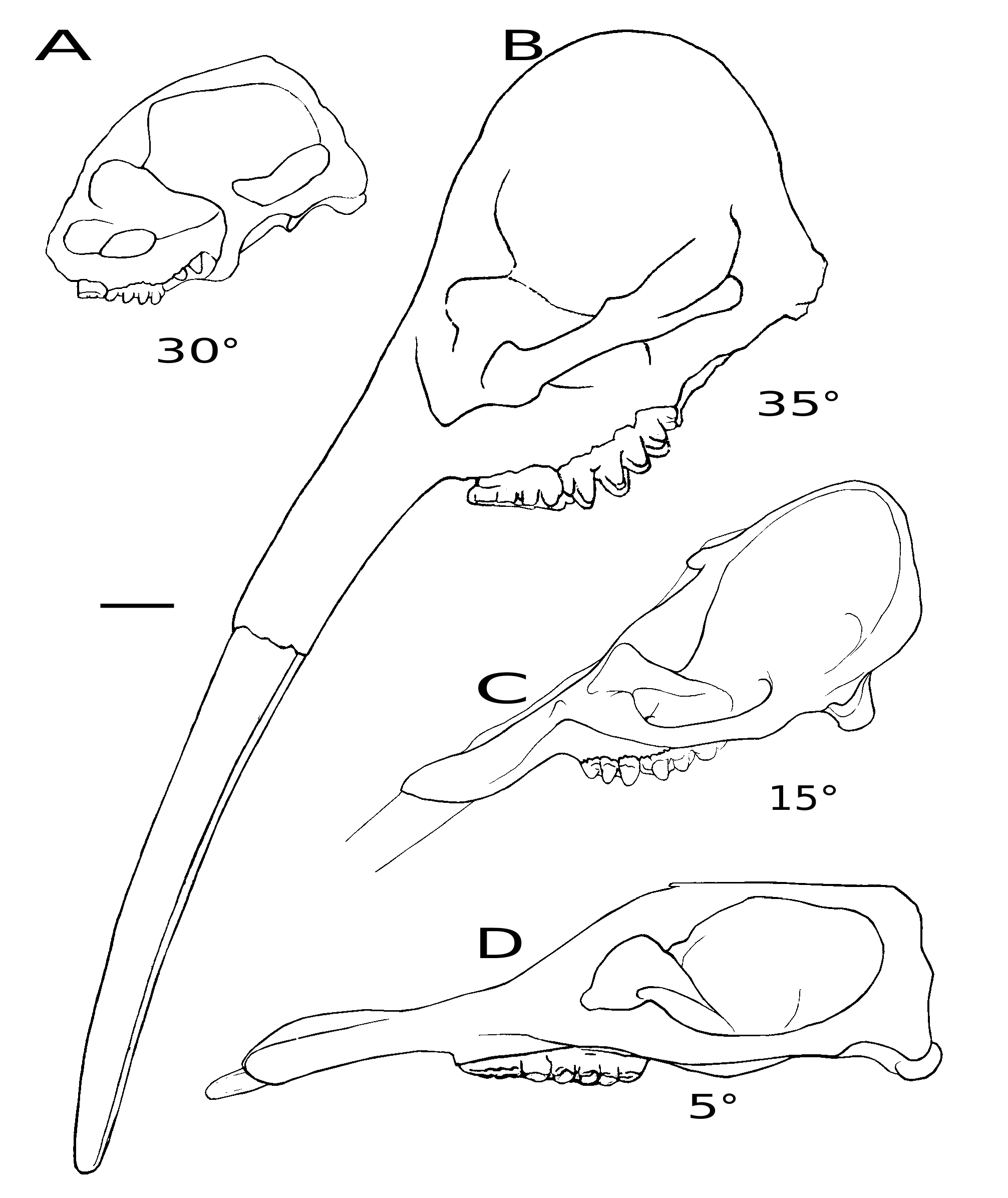
Comparisons. In dorsal view, the mandibular symphysis of K. robustus n. sp. does not expand laterally in the distal part. However, in most amebelodontines, the mandibular symphysis expands laterally in the distal part, especially in Platybelodon ( Fig. 5 View FIG A-I). This feature is correlated with the morphology of the lower tusks and will be further discussed below. In lateral view, the mandibular symphysis of K. robustus n. sp. is downwardly deflected. The deflection is greater than that in Gomphotherium and Platybelodon ( Fig. 5 View FIG J-L, N), and similar to that in Amebelodon , some Tetralophodon , and juvenile K. atticus ( Fig. 5M View FIG ; also see Schlesinger 1917; Barbour 1927; Mottl 1969; Ferretti et al. 2003; Konidaris et al. 2014). However, this deflection is significantly smaller than that of Stegotetrabelodon and some Tetralophodon ( Fig. 5 View FIG P-R).
The ramus of K. robustus n. sp. is almost perpendicular to the occlusal plan. This feature is a plesiomorphy as it is observed in Phiomia , and also in Konobelodon britti , Amebelodon fricki Barbour, 1927 , and Gomphotherium aff. steinheimense ( Fig. 5L, M View FIG ). In some longirostrine trilophodont taxa, such as in G. angustidens and Platybelodon grangeri , the ramus is more posteriorly inclined ( Fig. 5J, K View FIG ). The ramal shape, combined with the cranial shape, is correlated with the distribution of the jaw-closing muscles, and possibly represents different feeding behavior, which will be discussed below.
Cranium ( Fig. 6 View FIG ; Table 2)
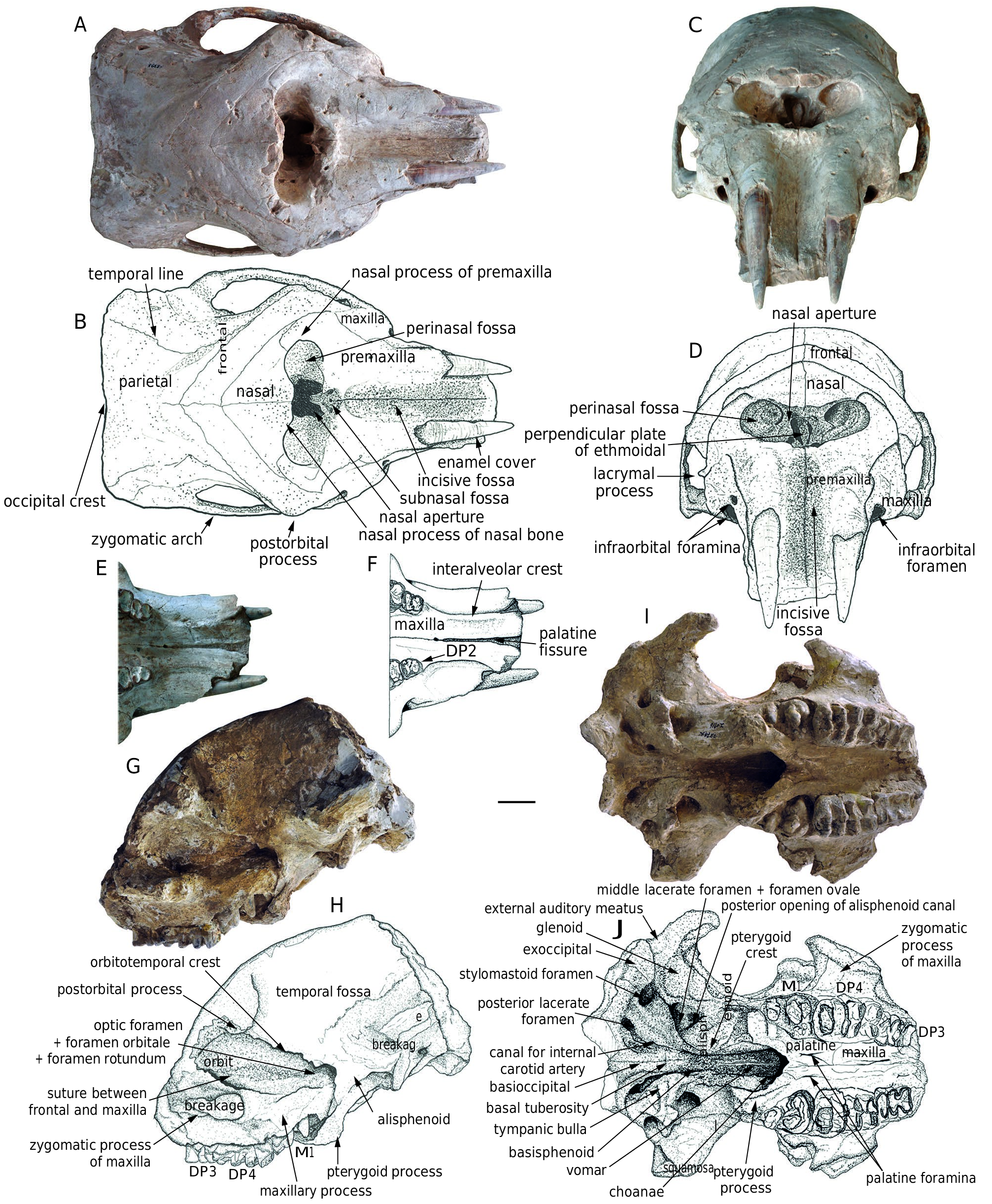
There are eight crania ( HMV 1904 , 1905 , 1456 , 1899 , 1901 , 1902 , 1910 , and 1906) in the study material, of which only HMV 1901 is a sub-adult (possessing P3-M1, and partial M2) and the others are juveniles. The descriptions in dorsal and anterior views, and of the anterior part in ventral view, are based on the paratype HMV 1910 ; the descriptions in lateral view and of the posterior part in ventral view are based on the paratype HMV 1904 .
Dorsal view ( Fig. 6A, B View FIG ). The posterior edge of the neurocranium (the occipital crest) is almost straight. The dorsal plate of the neurocranium is broad and flat, with a large distance between the two temporal lines. In juveniles, the sutures around the frontal bone are very clear. The frontal bone is narrow and extends anterolaterally to the upper rim of the orbits. The anterior edge of the frontal bone is in contact with the nasal and premaxillary bones, and its anterolateral corner is in contact with the maxilla. The nasal bone is triangular with a blunt nasal process. It extends laterally along the supe- rior rim of the nasal aperture and touching the nasal process of the premaxilla. The medial suture between the two nasal bones is also clear. The superior border of the nasal aperture is slightly posterior to the level of the two postorbital processes. The corpus of the premaxilla is long, with a strongly extending nasal process along the inferior and lateral borders of the nasal aperture. On the ventral border of the nasal aperture, the symphysis between the two premaxillae is prominent and encloses a small subnasal fossa (see Ferretti 2010), possibly for the insertion of the mesethmoid cartilage (see Tassy 1994a, b). The incisive fossa between the two premaxillae is narrow and deep. None of the crania have a complete anteriormost part of the alveoli, and thus we do not know whether the premaxilla is laterally expanded anteriorly. The zygomatic arch is not much laterally expanded from the cranium.
Anterior view ( Fig. 6C, D View FIG ). The nasal aperture is low and wide with well-developed perinasal fossae, forming a steplike structure (see Tassy 1994a, b). In the nasal aperture, the opening on the internal lateral surface is very clear. The
perpendicular plate of the ethmoidal bone in the medial position can also be observed. A small lacrymal process is located on the anterior rim of the orbit. In HMV 1910, the right infraorbital foramina are duplicated, as in trilophodont gomphotheres ( Tassy 1994b), and the two openings are very close. However, the left infraorbital foramen has only one opening, as in extant elephants ( Tassy 1994b, 2013). The infraorbital foramina aret located just anterior to the zygomatic process of the maxilla.
Ventral view ( Fig. 6E, F, I, J View FIG ). The basioccipital is strong. Anteriorly it is fused with the basisphenoid by a tough basal tuberosity. The basisphenoid tapers anteriorly and is fused with
a slim vomer that extends anteriorly into the choanae. The tympanic bulla is large and triangular, lateral to the basioccipital, and surrounded by a series of foramina: a medial and rounded canal for the internal carotid artery; a posterior, large, and irregular posterior lacerate foramen; and a lateral, large and rounded stylomastoid foramen. The middle lacerate and foramen ovale are confluent and located beneath the anterior margin of the bulla, with a large, rounded posterior opening of the alisphenoid canal anterior to the anterior edge of the bulla. The glenoid fossa is relatively flat and the exoccipital is strong and ventrally raised. Between the glenoid fossa and the exoccipital is a shallow groove for the external auditory channel; however, no postglenoid ledge is present. The choanae are oval with a sharp apex on the anterior rim. Lateral to the choanae, a strong pterygoid process is present with a long pterygoid crest posteriorly extending to the anteromedial angle of the tympanic bulla, in which the muscular process is embedded. The palate is narrow with a pair of slit-like palatine foramina. The zygomatic process of the maxilla is dorsally concave on its ventral surface. Two interalveolar crests converge in the middle. The anterior palatine fissure is prominent.
Lateral view ( Fig. 6G, H View FIG ). The neurocranium is moderately domed. The temporal fossa is large and not very anteroposteriorly compressed. The basicranium is moderately erected. In HMV 1904, although broken, the occipital condyle seems
posteroventrally protruded. The orbitotemporal crest extends posteroinferiorly to reach the anterior edge of the alisphenoid. A large fissure is located beneath the anterior margin of the alisphenoid, in which the optic foramen, the foramen orbitale, and the foramen rotundum are present. The anterior edge of the alisphenoid turns anteroinferiorly to the pterygoid process, and wraps the posterior end of the posterodorsally erected maxillary process in which an embryo cheek tooth grows. The orbit, in which the transverse suture between the frontal and the maxilla clearly runs from the anterior rim to the anterior margin of the orbitotemporal crest, is rounded. The maxilla inferior to the zygomatic process is low. The occipital surfaces of all known specimens are broken.
Comparisons. The juvenile cranium of K. robustus n. sp. has a relatively domed neurocranium and an erected basicranium ( Fig. 7A View FIG ). In the juvenile cranium of K. atticus from Pikermi ( Greece), the neurocranium is less domed and the basicranium is only moderately erected ( Konidaris et al. 2014). Kovachev (2004) reported an adult cranium of K. atticus from the East Maritsa Basin ( Bulgaria), and its basicranium is also erected like that of K. robustus n. sp. However, the cranium figured in Kovachev (2004: fig. 1 in pl. 2) shows a concave dorsal outline of the neurocranium in lateral view. We do not know whether this feature is the result of ontogeny, intraspecific variation, or merely an uncorrected reconstruction of the specimen. Nevertheless, a moderately domed neurocranium, an anterioposterioly compressed temporal fossa, and a moderately erected basicranium have also been found in Paratetralophodon hasnotensis (Osborn, 1929) from the Dhok Pathan Formation, Siwaliks, Pakistan (nothing is known about the mandible in this taxon) ( Tassy 1983b; see Fig. 7B View FIG ). Interestingly, in the paratype HMV 1909, duplicated infraorbital foramina were found on its right side (as in trilophodont gomphotheres including amebelodontines) but a single foramen on its left side (as in true elephants) ( Tassy 1994b; see Fig. 6C, D View FIG ). In Gomphotherium angustidens , the neurocranium is only slightly domed and the basicranium is also slightly erected ( Fig. 7C View FIG ), but these features are even less developed in known crania of amebelodontines such as Platybelodon grangeri ( Fig. 7D View FIG ).
Tusks ( Figs 3 View FIG , 4 View FIG , 6 View FIG , 8 View FIG ; Tables 3, 4)
Upper tusk ( Figs 6 View FIG A-F; 8A, B). Three crania ( HMV 1910, 1906, and 1901) possess paired upper permanent tusks and
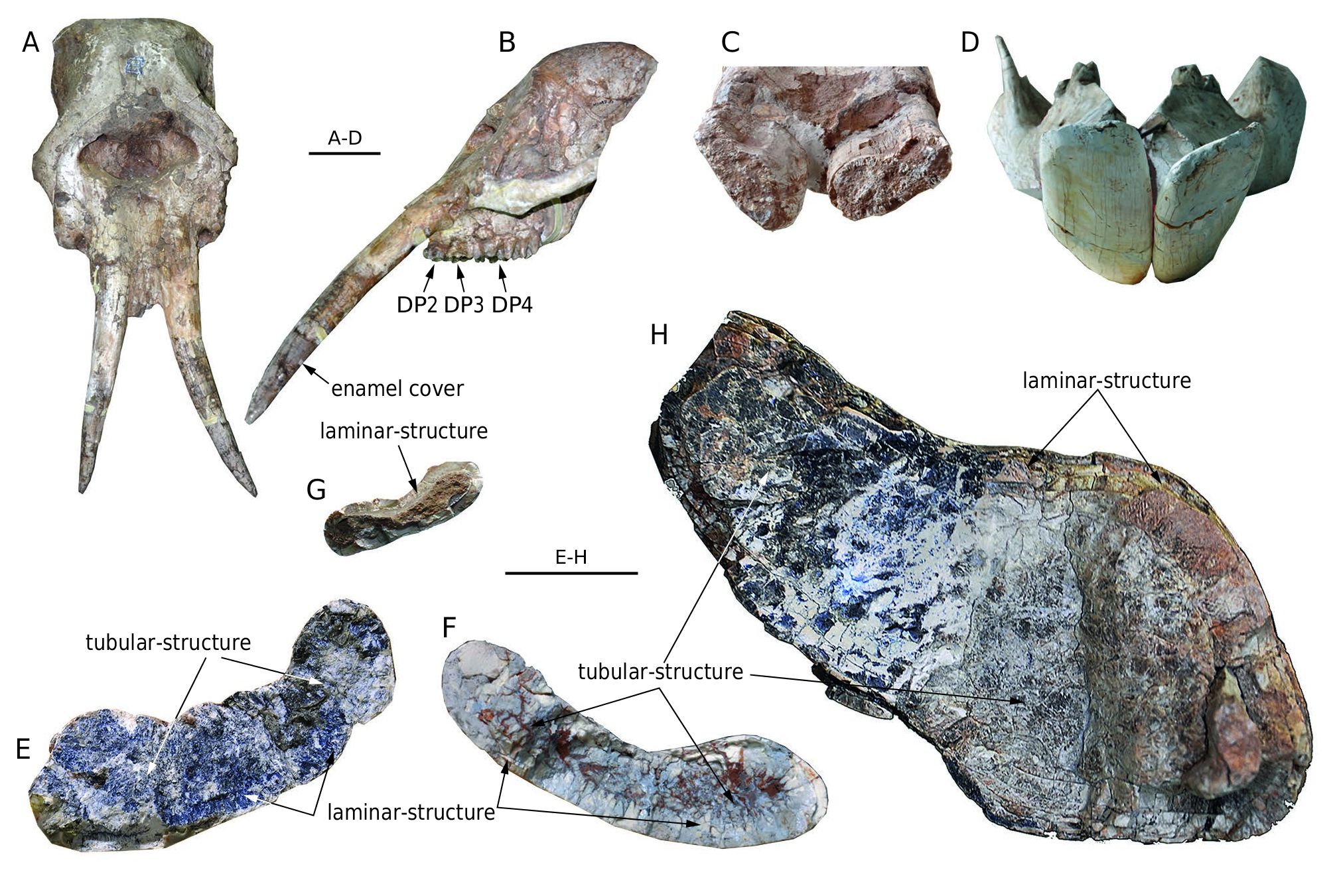
HMV 1879.3 is an isolated upper tusk fragment. The upper tusk is strong, oval in cross-section, and clearly ventrally curved. At young ontogenetic stages (younger than dental age IV), the two tusks are almost parallel and have enamel covers ( Fig. 6 View FIG A-F). In older ontogenetic stages (older than dental age V), they are strongly diverging. There is no enamel band on the lateroventral surface of the tusk even when the apical part (which is 145 mm in length from the tip in the left tusk of HMV 1906) is covered by enamel ( Fig. 8A, B View FIG ). The tip of the tusks is simply polished.
Lower tusk ( Figs 3 View FIG ; 4 View FIG ; 8 View FIG C-G). As well as the holotype ( HMV 0004), eight mandibles ( HMV 1887, 0001, 0003, 0011, 1903, 1909, 1907, and 1908) in the study material possess paired lower permanent tusks, and one ( HMV 1893) has a left lower permanent tusk. Another five ( HMV 1787, 1893, 1861, 1879.1, and 1879.2) are fragmentary segments.
The cross-section of the permanent tusk is flattened and dorsally concave without a ventral groove ( Fig. 8 View FIG E-G). In juvenile individuals, the tusk is narrow, tapers anteriorly, and has an enamel cover on the tip with many enamel buds on the anterolateral edge. In the adult type specimen HMV 0004, the tusk length is estimated as c. 900 mm, including the part in the alveolus. The exposed length is much greater than the symphyseal length. In lateral view, the tusk is dorsally bent. In dorsal view, the two tusks are divergent. The medial surface is rounded. At the crosssection of the alveoli, the long axes of the two tusks are oblique medioventrally at an angle ( Fig. 8C View FIG ). At the apical ends, the long axes of the two tusks are more horizontal ( Fig. 8D View FIG ), caused by the outward twisting of the tusks. Narrow wear facets are present along both dorsal and ventral sides of the apical end of the tusk, forming a relatively sharp anterior edge.
A tubular structure enclosed by one or several concentric laminae is visible in the cross-section beyond a young ontogenetic age. In the cross-section at the level of the alveoli ( Fig. 8E View FIG ), the tubules are very thin (estimated tubule diameter less than c. 1 mm in HMV 1887); and in the cross-section in the middle of the tusk, tubules become thicker (estimated tubule diameter c. 1.5 mm in HMV 0011) ( Fig. 8F View FIG ). At a young ontogenetic stage, the tubular structure appears not to be present ( Fig. 8G View FIG ).
Comparisons. No upper tusk of adult K. robustus n. sp. is known. In juvenile individuals, an enamel cover is present on the apical part of the upper tusks. It can be assumed that the enamel cover will be used off, as in K. atticus from Pestszentlőrincz, Hungary ( Schlesinger 1922). However, unlike K. britti , no lateral enamel band is present.
The upper tusks of K. robustus n. sp. are apparently morphologically distinct from those in the hypothetical cranial reconstruction of K. atticus from Pestszentlőrincz ( Schlesinger 1922). The upper tusks of K. robustus n. sp. are divergent in dorsal view and ventrally bent in lateral view ( Fig. 8A, B View FIG ). However, we do not know the eventual orientation of these tusks in adults K. robustus n. sp. It is possible that the reconstruction of the upper tusks by Schlesinger (1922) is not accurate, and the orientation of the upper tusks in K. atticus was similar to that found in juvenile K. robustus n. sp. from the Linxia Basin.
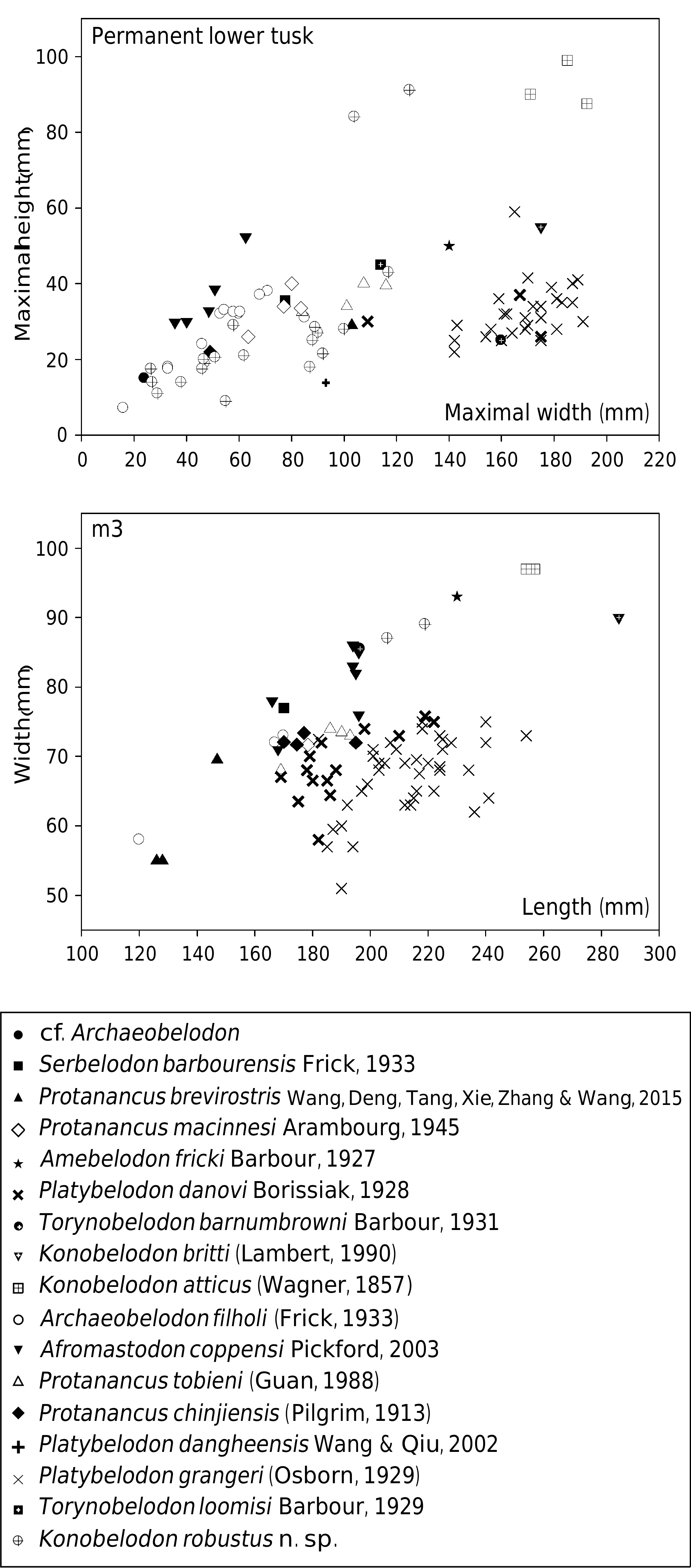
The lower tusk of K. robustus n. sp. is long and flattened, similar to those of K. atticus and K. britti . The width of the cross-section is much smaller than those of K. britti and K. atticus ( Fig. 9 View FIG ). The medial surface of the lower tusk is rounded, unlike the flattish medial surface in some amebelodontines such as Platybelodon . In addition, the dimensions of the lower tusk cross-section of Torynobelodon loomisi Barbour, 1929 do not fall into the range of Platybelodon , in contrast to that of T. barnumbrowni Barbour, 1931 , which falls into the range of Platybelodon .
The cross-section of the lower tusk of K. robustus n. sp. is dorsally concave and ventrally convex, and is without a ventral groove. This feature is similar to K. britti . However, in K. atticus , the cross-section of the lower tusk is flattened pyriform shape with a ventral and dorsal concavity. A tubular structure enclosed by dentinal layer(s) in cross-section is distributed throughout the entire length of the lower tusk of K. robustus n. sp., except in the most juvenile permanent lower tusks. In the proximal cross-section ( Fig. 8E View FIG ), the tubular structure is very fine (estimated tubule diameter less than c. 1 mm), and is not easily to be distinguished from the dentinal matrix, whereas in the medial cross-section ( Fig. 8F View FIG ), the tubular structure appears clearer and the dentinal tubules are thicker (estimated tubule diameter c. 1.5 mm). The increase in tubule thickness basiapically is similar to that of K. atticus and K. britti , but, in general, the tubules in K. robustus n. sp. are thinner and unclearer than those of the other two species. Tubular structure is a derived feature not present in primitive elephantiforms, and, logically, thicker tubules are more derived than thinner tubules. Furthermore, the stratigraphic range of K. robustus n. sp. ( MN 9- MN 10) is earlier than K. atticus ( MN 11- MN 13) and K. britti (Hemphillian, c. 7 Ma) ( Lambert 1990; Konidaris et al. 2014). Therefore, K. robustus n. sp. is possibly more ancestral than the other species of the genus Konobelodon .
The two lower tusks of K. robustus n. sp. are divergent in situ. They are more divergent in adults than in sub-adults and juveniles ( Figs 3 View FIG , 4 View FIG ). Thus this feature is strongly correlated with ontogeny. In juvenile K. atticus the two lower tusks are slightly convergent ( Markov et al. 2014: fig. 1). In juvenile K. robustus n. sp., the lower tusks are clearly divergent. The medial edge is thicker than the lateral edge. The paratype of K. britti , an isolated lower tusk, was identified as a right tusk by Lambert (1990: fig. 3). He also stated that the lateral edge is far thicker than the medial edge ( Lambert 1990: 1035). However, comparing the states in K. robustus n. sp. and in adult K. atticus , we believe that the lower tusk of the paratype of K. britti is actually a left tusk.
We have mentioned that, unlike the other amebelodontines, the mandibular symphysis of K. robustus n. sp. does not expand laterally in the distal part. This feature is correlated with the morphology of the lower tusks. In K. robustus n. sp., at the level of the alveoli, the two long axes of the tusk cross-section are oblique medioventrally, forming an angle ( Fig. 8C View FIG ). Related to that, the mandibular symphysis is high at the base. A narrow, high symphysis helps reducing the twisting stress within the symphysis when an external rotation torque is exerted on the distal part of the lower tusks, as the animal may use its lower tusks for digging as part of the feeding behaviour. To compensate, the two tusks are outwardly twisted, and thus almost horizontal at the apical end ( Fig. 8D View FIG ), as in the other amebelodontines.
The exposed length of the lower tusks in adult individuals of K. robustus n. sp. is longer than the symphyseal length ( Fig. 5F, N View FIG ). Because this feature is observed only on one adult specimen, it cannot be ruled out if it is sex-related. This feature is rare in proboscideans, except in stegotetrabelodonts ( Fig. 5Q, R View FIG , also see Petrocchi 1943; Maglio 1973; Tassy 1999). This character is probably the result of convergent evolution and is also strongly correlated with ontogeny, because in juvenile individuals of K. robustus n. sp. the exposed length of the lower tusks is much shorter than the symphyseal length and in sub-adults they are nearly equal in length ( Fig. 4 View FIG ).
Cheek teeth ( Fig. 10 View FIG ; Tables 5; 6)
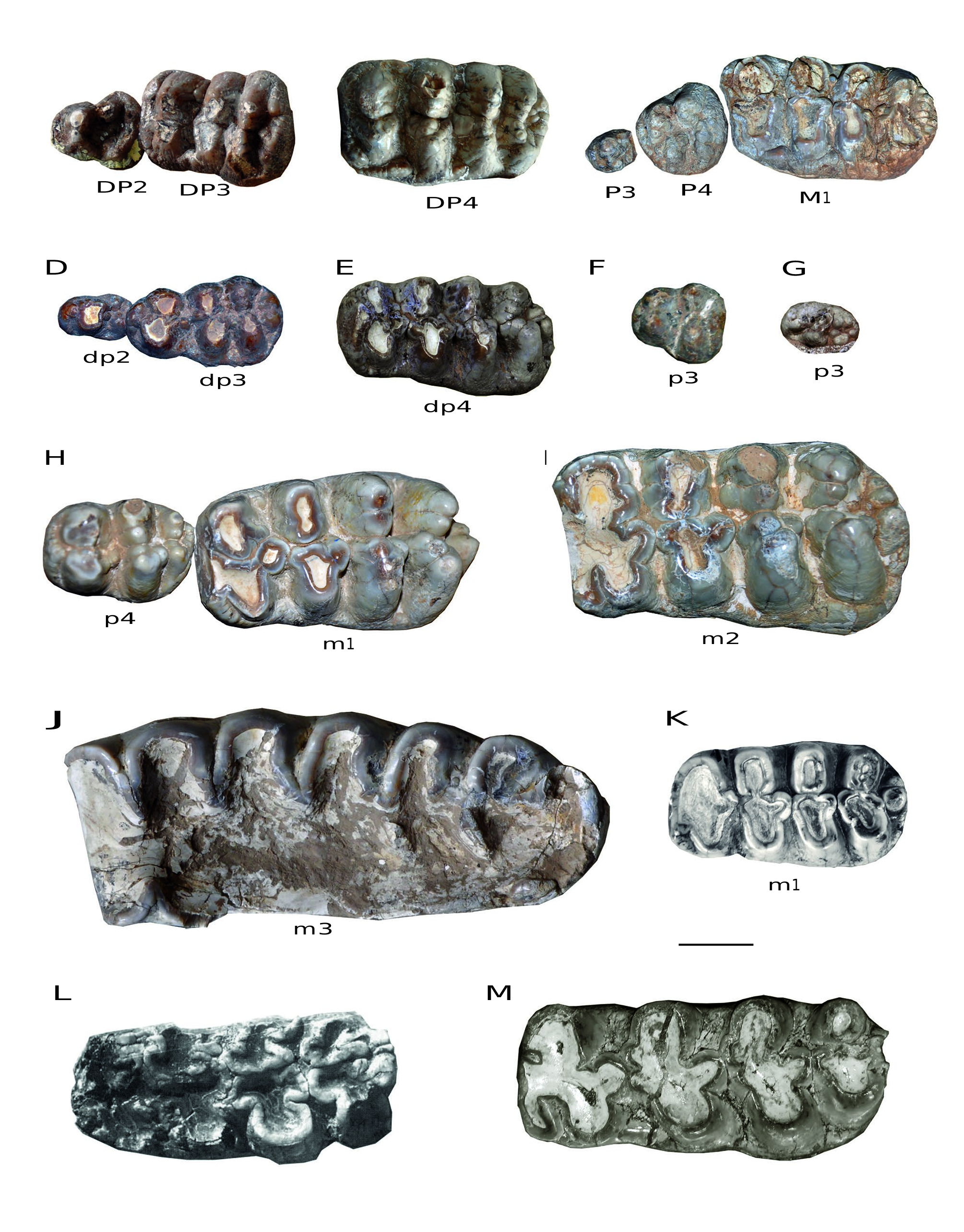
DP2 ( Fig. 10A View FIG ). There are 12 DP2s [ HMV 1889 (l.), 1905 (r.), 1456 (l. + r.), 1899 (l. + r.), 1901 (l. + r.), 1910 (l. + r.), and 1906 (l. + r.)] in the study material. The DP2 is triangular. The paracone and the protocone are connected with each other; the former is larger and higher than the latter. The metacone and hypocone are small and separated. The cingulum is strong, and surrounds at least the anterior and posterior margins of the tooth.
DP3 ( Fig. 10A View FIG ). There are 18 DP3s [ HMV 1884 (l. + r.), 1889 (l.), 1904(l. + r.), 1905 (r.), 0001 (l. + r.), 1456 (l. + r.), 1899 (l. + r.), 1901 (l. + r.), 1910 (l. + r.), and 1906 (l. + r.)] in the study material. The DP3 is rectangular and trilophodont. Although anterior and posterior pretrite central conules are small, pretrite trefoils are visible at least on the first two lophs. In these two lophs, the anterior and posterior pretrite central conules are almost of equal dimensions. The last two lophs are slightly anteriorly curved. The third loph is enlarged with marked ento- and ectoflexus. On the second loph, the metacone (posttrite) is posterior to the hypocone (pretrite); thus, the connection between the successive lophs in the two interlophs is the anterior posttrite half-loph with the next posterior pretrite half-loph. Ptychodonty is present and the posterior cingulum is strong. In some cases, the posterior cingulum is as strong as a fourth loph.
DP4 ( Fig. 10B View FIG ). There are eight DP4s [ HMV 1884 (l. + r.), 1889 (l.), 1904(l. + r.), 1905 (r.), and 1901 (l. + r.)] in the study material. The DP4 is rectangular and tetralophodont. Complete pretrite trefoils are developed at least on the first two lophs and slightly anterior curvature is often visible on the last two lophs. The cusps on the lophs and central conules tend to be subdivided into smaller ones and are aligned along the lophs (especially for the posterior lophs), and thus are crest-like. The interlophs are anteroposteriorly compressed. Ptychodonty and the cingulum seem to be reduced relative to the DP3.
P3 ( Fig. 10C View FIG ). There is only one P3 [ HMV 1902 (l.)] in the study material. The P3 is small, oval with a strong cusp in the centre, and cingula are surrounding the tooth.
P4 ( Fig. 10C View FIG ). There are only two P4s [ HMV 1902 (l. + r.)] in the study material. The P4 is quadrate to oval. The protocone is trifoliate. The paracone is anteriorly oblique to the midline, and higher than the protocone. The metacone and hypocone form a posterior loph. The paracone, metacone and hypocone are subdivided. The anterior and posterior cingula are strong.
M1 ( Fig. 10C View FIG ). There are only two M1s [ HMV 1902 (l. + r.)] in the study material. The M1 is rectangular and tetralophodont. The morphology is similar to that of the DP4. Pretrite trefoils are developed on the first three lophs and chevroning is visible on the fourth loph. Cingula are present on the anterior, posterior, and lingual rims of the tooth.
dp2 ( Fig. 10D View FIG ). There are nine dp2s [ HMV 1892 (r.), 1893 (r.), 1456 (l. + r.), 1900(l.), 1909 (l. + r.), and 1908 (l. + r.)] in the study material. The dp2 is more slender than the DP2. The protoconid and the metaconid are connected with each other, and the latter is higher. The hypoconid and the entoconid are small and separated. The posterior cingulid is reduced.
dp3 ( Fig. 10D View FIG ). There are 12 dp3s [ HMV 1892 (r.), 1893 (r.), 0003 (l. + r.), 1456 (l. + r.), 1900 (l. + r.), 1909 (l. + r.), and 1908 (l. + r.)] in the study material. The dp3 is composed of three lophids, the first one being transversely narrower than the last two. Complete pretrite trefoils are visible at least on the first lophid and the posterior pretrite central conule is larger than the anterior one. On the second lophid, the entoconid (posttrite) is anterior to the hypoconid (pretrite). The second interlophid is anteroposteriorly wider than the first. In the first interlophid, the first posterior pretrite central conule is connected with the second posttrite half-lophid; whereas, in the second interlophid, the second and the third pretrite half-lophids are connected. Ptychodonty and the cingulid are reduced, relative to the DP3. In some cases, the posterior cingulid is as strong as a fourth lophid.
dp4 ( Fig. 10E View FIG ). There are eight dp4s [ HMV 0003 (l. + r.), 1903 (l. + r.), 1909 (l. + r.), and 1907 (l. + r.)] in the study material. The dp4 is similar to the DP4, but narrower. The lophids are more oblique anterolingually than those of the DP4. The central conules are more developed than those in the DP4. Complete pretrite trefoils are developed at least on the first three lophids and chevroning is often visible on the last lophid. On the first two lophids, the posterior pretrite central conules are generally larger than the anterior ones. The cusps on the lophids also tend to be subdivided, but the tendency is not as strong as that in the DP4. The posterior cingulid is often composed of two cusps.
A B C
m 2 m 2
p3 ( Fig. 10F, G View FIG ). There are five p3s [ HMV 1888 (l.), 1903 (l. + r.), and 1907 (l. + r.)] in the study material. The p3 from the higher horizon (the Dashenggou fauna) is triangular ( Fig. 10F View FIG ), as in some other tetralophodont gomphotheres, e.g., Tetralophodon longirostris and “ T. exoletus ” ( Schlesinger 1917; Hopwood 1935). The protoconid and metaconid are connected with each other, whereas the hypoconid and entoconid are separated. The cingulid is on the anterior and posterior edges. However a p3 from the lower horizon (the Guonigou fauna) is oval, although not fully erupted ( Fig. 10G View FIG ).
p4 ( Fig. 10H View FIG ). There are only two p4s [ HMV 0011 (l. + r.)] in the study material. The p4 is oval, composed of two complete lophids and a third forming lophid. The protoconid is trifoliate, and the hypoconid lacks the anterior central pretrite conule. The entoconid is subdivided into two cusps. The “third lophid” is also composed of a row of (c. 4) conelets, each of which is smaller than that of the second lophid. Anterior and posterior cingulids are also developed.
m1 ( Fig. 10H View FIG ). There are six m1s [ HMV 1887 (l. + r.), 0011 (l. + r.), and 1907 (l. + r.)] in the study material. The m1 is rectangular and tetralophodont. Pretrite trefoils are developed at least on the first three lophids and chevroning is present on the fourth lophid. The pretrite half-lophids are mediolaterally elongated or subdivided, especially the posterior two lophids. On the first two lophids, the posterior pretrite central conules are generally larger than the anterior ones. The posttrite half-lophids are generally simple, with a main cuspid and a mesoconelet. The anterior cingulid is weak, and the posterior cingulid is composed of two cuspids. The cementum in the valleys is weak.
m2 ( Fig. 10I View FIG ). There are only two m2s [ HMV 0001 (l. + r.)] in the study material. The m2 is generally similar to the m1; however, anterior and posterior posttrite central conules are developed on the first two half-lophids. The tooth also shows a weak tendency to anancoidy and cementodonty.
m3 ( Fig. 10J View FIG ). The only m3s on the holotype HMV 0004 are fully worn. The m3 is composed of six lophids and a not fully developed seventh lophid.
Comparisons. The P3 of K. robustus n. sp. is small and oval. It is more regressive than that in Tetralophodon longirostris ( Schlesinger 1917) , in which the P3 is more complex with a quadrate shape. The p3 of K. robustus n. sp. of the lower ho- rizon (the Guonigou Fauna) is oval; while that of the upper horizon (the Dashenggou Fauna) is triangular. An oval-shaped p3 has been reported in trilophodont gomphotheres ( Tassy 1985; Wang & Qiu 2002; Göhlich 2010; Wang et al. 2013c). Therefore, a triangular p3 is a derived feature of tetralophodont gomphotheres. Furthermore, p3 is lost in Platybelodon grangeri ( Wang et al. 2013b) .
The cheek teeth of K. robustus n. sp. are similar to the typical tetralophodont gomphotheres: three loph(id)s in DP3/ dp3, four loph(id)s in the intermediate cheek teeth, and 6-7 lophids in m3. The m3 is close in size with that of K. atticus but smaller than that of K. britti ( Fig. 9 View FIG ). The tooth morphology of K. robustus n. sp. and K. atticus resembles that of Tetralophodon logirostris more than that of K. britti and Platybelodon
Specimen HMV 1881 Maximal length 81 Maximal width (between the two wings) – Maximal height 168
grangeri , although all of them have a tetralophodont m2. Like in Tetralophodon logirostris ( Fig. 10K View FIG ) and in K. atticus , the interloph(id)s in K. robustus n. sp. are anterioposteriorly com- pressed, and secondary trefoils and pseudo-anancoidy are not marked. In lower molars of K. britti ( Lambert 1990; Lucas & Morgan 2008), secondary trefoils and pseudo-anancoidy are very pronounced ( Fig. 10L View FIG ). In Platybelodon grangeri , only an advanced form of this taxon (in the Tamuqin fauna of the upper Tunggur Formation, see Wang et al. 2013b) possesses a tetralophodont m2, whereas the m1 and dp4 are still trilophodont. Even in the tetralophodont m2 of Platybelodon grangeri , the fourth lophid is incipient, the interlophids are wider in the anteroposterior dimension, and the contour is narrower than that in K. robustus n. sp. ( Fig. 10M View FIG ).
Postcranial bones ( Figs 11-15 View FIG View FIG View FIG View FIG View FIG ; Tables 7 -14)
Atlas ( Fig. 11 View FIG A-C; Table 7). The only atlas ( HMV 1881) is elliptical in cranial and caudal views, and compressed craniocaudally. The vertebral foramen is sub-rectangular to oval with a transverse constriction in the medial part. The dorsal arch is thin and low. Most of the transverse processes are broken, and only part of the right one remains, with a rounded opening of the transversal foramen. In cranial view, the articular surface for the occipital condyle is broad, concave, and reniform. In caudal view, the articular surface for the corpus of the axis is sub-quadrate with a marked dorsomedial angle. The facet for the dens of the axis is at the ventromedial part of the ventral arch, and is oblique dorsocaudally. In dorsal view, two interconnected lateral vertebral foramina are located on each side of the dorsal arch with a shallow groove extending from the opening of the lateral vertebral foramen to the cranial opening of the transversal foramen.
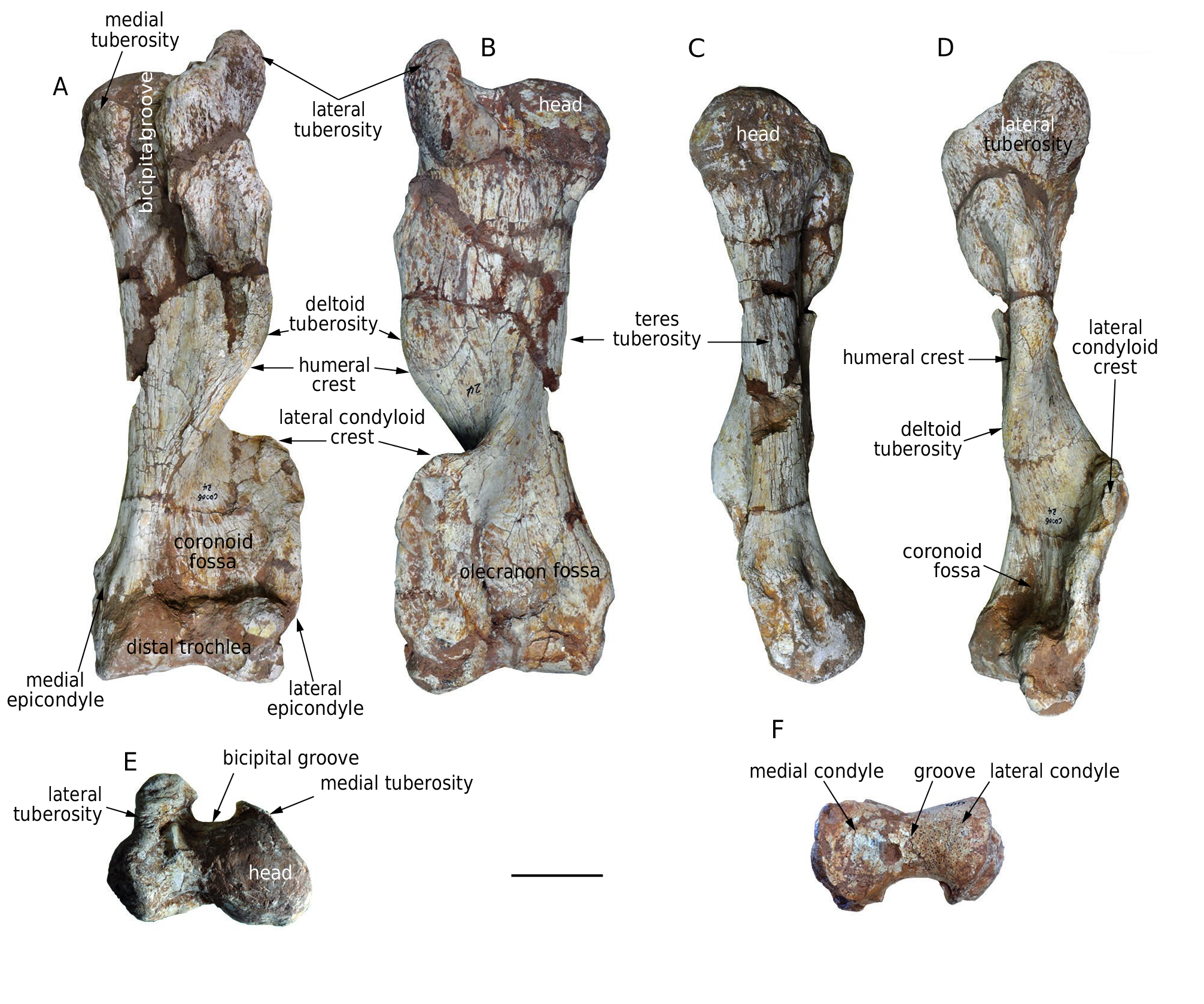
Humerus ( Fig. 12 View FIG A-F; Table 8). There are three humeri [ HMV 1882 (l.), 1891 (l.), and 1911 (l.)] in the study material. The humerus is very thick. The middle shaft is thin but strongly expanded proximally and distally, and the shaft is strongly twisted clockwise in proximal view for the left humerus. The humeral crest extends distally from the lateral tuberosity and protrudes in the middle, forming a strong deltoid tuberosity, then turns mediocranially to form the medial border of the coronoid fossa. The lateral condyloid crest initially extends strongly laterally and then makes an acute turn distally, forming an almost right angle and enclosing both a broad coronoid fossa cranially and a broad olecranon fossa caudally. The teres tuberosity is weak. Proximally the humeral head is hemispherical, and the lateral tuberosity is somewhat higher than the head. The medial tuberosity is small. The bicipital groove between the medial and lateral tuberosities is very deep. Distally, the medial condyle of the trochlea is larger than the lateral one, with a wide groove between them. This groove is shallow on the cranial face and deep on the caudal face of the distal trochlea. The lateral depression between the lateral condyle and the epicondyle is more prominent than the medial depression, and the medial epicondyle is more proximally positioned than the lateral one.
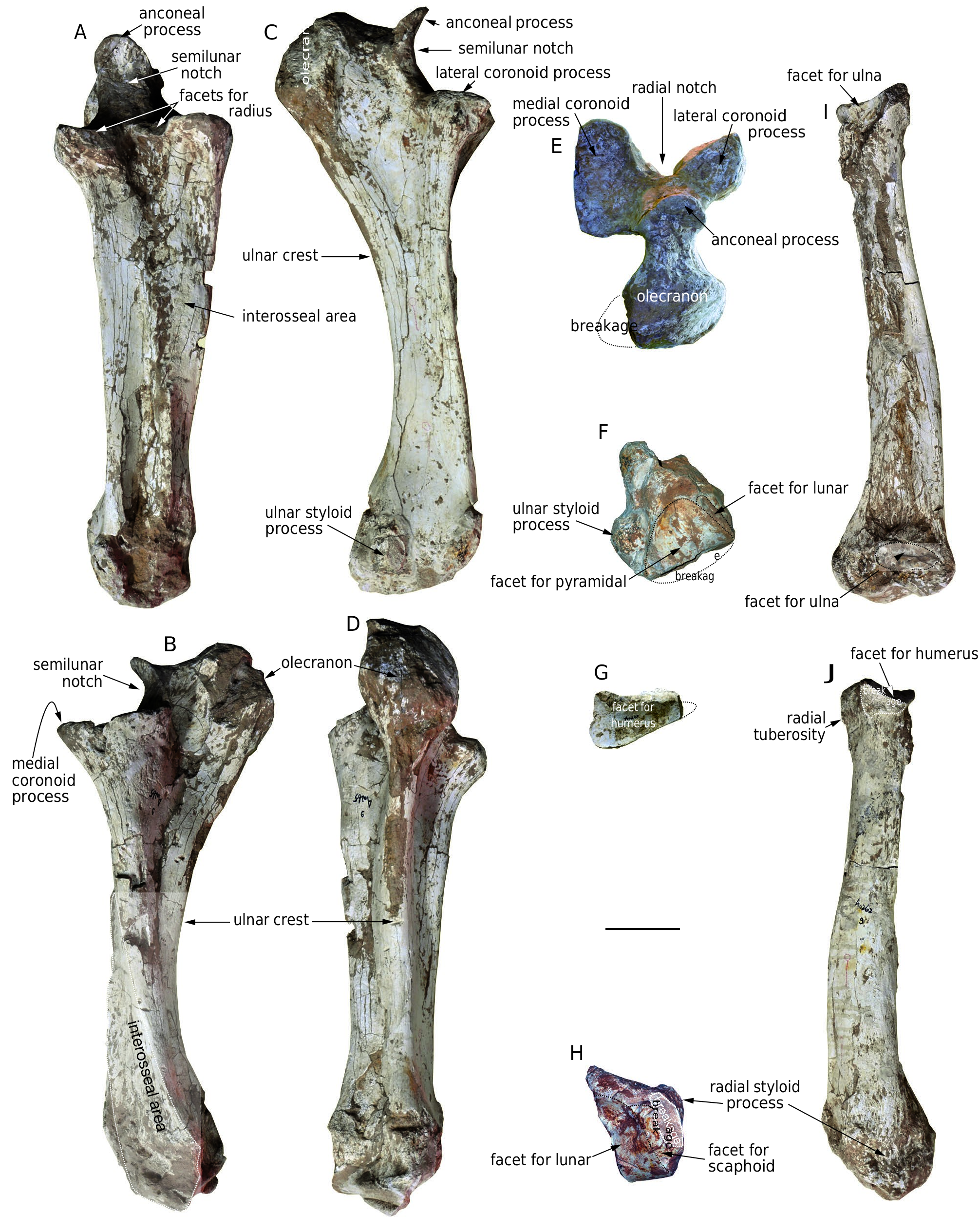
Ulna ( Fig. 13 View FIG A-F; Table 9). There are three ulnae [ HMV 0539 (?r.), 1886 (l.), and 1895 (l.)] in the study material. The ulna is moderately thick. The cross-section of the shaft is triangular in the proximal part, becoming rectangular in the distal part. In dorsal view, the shaft slightly tapers distally. In lateral and medial views the corpus is volarly concave. In volar view, the
ulnar crest is strong and extends throughout the bone. Proximally, the olecranon is swollen, with the medial side more swollen than the lateral side in proximal view. The anconeal process is hook-like and the tip is almost at the same level as the olecranon. The semilunar notch is dorsally concave and smooth. The coronoid process is divided into two lobes by a deep triangular radial notch, and the medial lobe is larger than the lateral one. On both the dorsomedial and dorsolateral sides of the radial notch is a small semilunar facet for the radius. Distally, the facet for the radius is not clear. In distal view, the main facet for the pyramidal is triangular. A small vertical facet meets the dorsomedial side of the pyramidal facet for the lunar. On the lateral side of the distal extremity, the ulnar styloid process is moderately raised.
Radius ( Fig. 13 View FIG G-J; Table 10). The shaft of the only radius [ HMV 1896 (r.)] is compressed dorsovolarly and turns distomedially. The mediovolar face of the shaft is concave and rough, facing the corpus of the ulna. The laterocaudal face is convex and smooth. Proximally, the radial tuberosity is moderately developed. The proximal facet for the humerus is triangular and the sharpest angle is laterally oriented. The medial edge is almost perpendicular to the dorsal edge, and two lunar facets for the proximal notch of the ulna are present on the proximal extremity of the bone and meet the proximal facet along its medial and laterovolar edges. Distally, the styloid process is weak. The distal articular surface is convex, irregularly tetragonal, and with a sharp dorsolateral angle. The ventral part of the articular surface is divided into two parts by a weak crest, of which the small, medial one is for the scaphoid and the large, lateral one for the lunar.
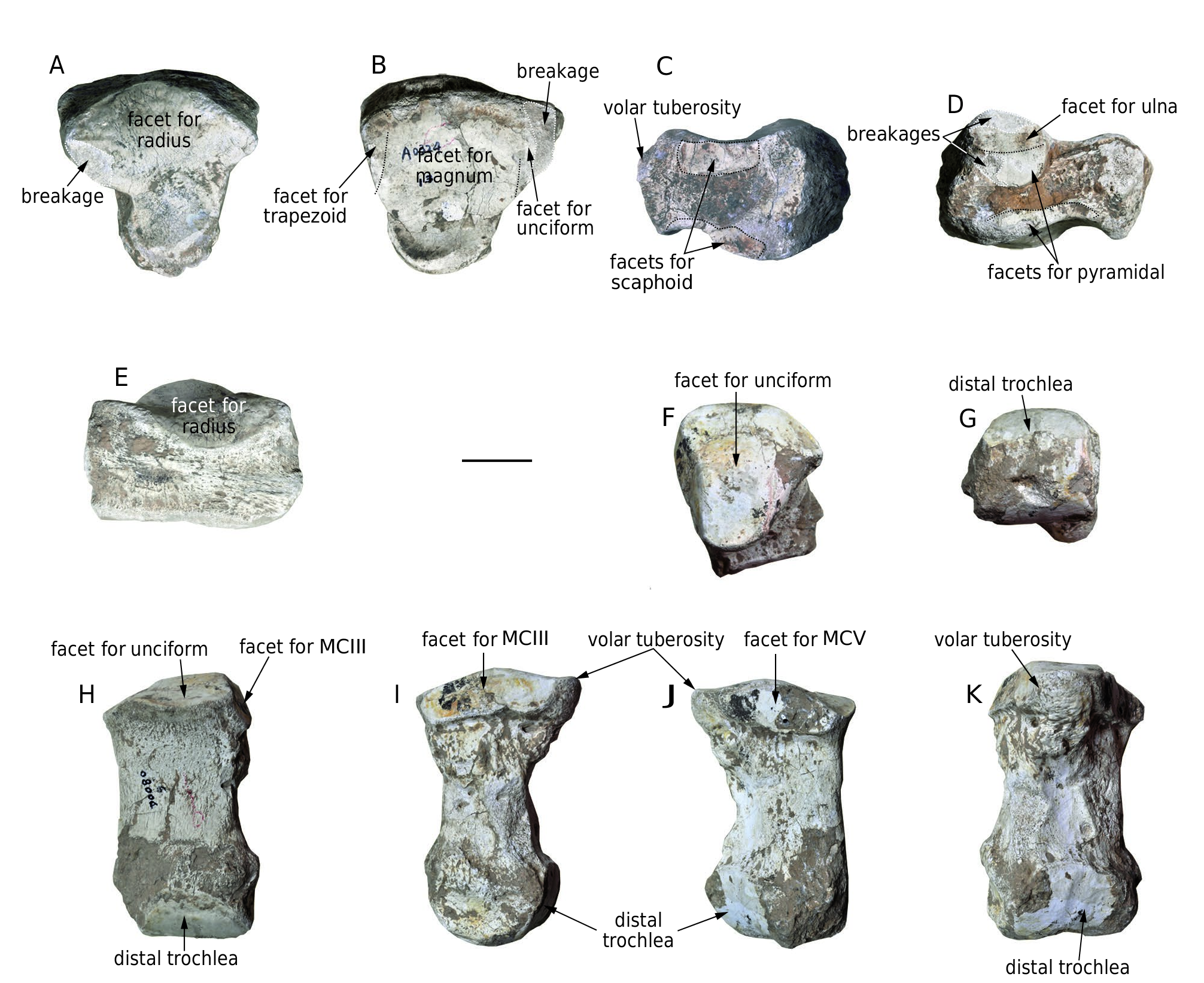
Lunar ( Fig. 14 View FIG A-E; Table 11). The only lunar [ HMV 1890 (l.)] is flat and stout. In proximal and distal views, the shape is triangular. The proximal facet for the radius is saddle-shaped, convex in the dorsal part and concave in the volar part. There is only a small, elliptical facet for the ulna (damaged) along the dorsolateral border of the main facet. The distal facet is concave, most of which is for the magnum, with only a small area of the anterior margin of both the medial and lateral sides joining to the trapezoid and the unciform, respectively. However, there is no clear boundary between these parts of the facet. In dorsal view, the shape is rectangular with a rough anterior face. In medial and lateral views, the dorsal half of the bone is much swollen. The medial facets for the scaphoid are along the proximal and distal margins of the bone and are separated by a notch. Along this notch, the proximal facet is much larger than the distal facet. The lateral facets are also divided into proximal and distal facets by a notch. The proximal facet is sub-circular, dorsally positioned, and is further subdivided by a weak transverse crest, the proximal part of which is for the ulna. The distal part of the proximal facet, and the distal facet along the distal margin of the bone is for the pyramidal. The volar tuberosity is strong.
Metacarpal IV ( Fig. 14 View FIG F-K; Table 12). The only metacarpal IV [ HMV 1893 (l.)] is also thick. The shaft is relatively short, with slight anticlockwise torsion in proximal view. It is triangular in cross-section and only slightly expanded in its extremities. The facet for the unciform takes up almost the entire proximal surface. This facet is triangular and convex. The proximal medial facet for metacarpal III and the proximal lateral facet for metacarpal V are dorsovolarly elongated; both meet the proximal facet with a crest. The proximal medial facet is longer than the lateral. The volar tuberosity is large and protruding. The distal extremity is almost equal in width to the proximal one. The width at the epicondyles is wider than that of the trochlea. In distal view, the trochlea is convex dorsally and straight or slightly concave volarly. The volar keel of the trochlea is almost absent.
HMV 1890 Specimen left Maximal width 150 Maximal depth 149 Maximal height 89 Width of the facet for radius 137 Depth of the facet for radius 130 Width of the distal facet 128 Depth of the distal facet 129 Depth of the proximal facet of the medial side 70.5 Height of the proximal facet of the medial side 28 Depth of the distal facet of the medial side 67 Height of the distal facet of the medial side 31 Depth of the proximal facet of the lateral side 55.5 Height of the proximal facet of the lateral side 56 Depth of the distal facet of the lateral side 80 Height of the distal facet of the lateral side 16
HMV 1893 Specimen left Maximal length 171 Minimal width of the middle shaft 76 Minimal depth of the middle shaft 54 Minimal perimeter of the middle shaft 220 Proximal width 100 Proximal depth 104 Width of the proximal facet for pyramidal 89 Depth of the proximal facet for pyramidal 102.5 Depth of the proximal facet for metacarpal III 88 Length of the proximal facet for metacarpal III 34.5 Depth of the proximal facet for metacarpal V 78.5 Length of the proximal facet for metacarpal V 28.5 Distal width 103
Distal depth 82 Width of the distal trochlea 82.5 Thickness index = Minimal width of the middle 0.444 shaft/Maximal length
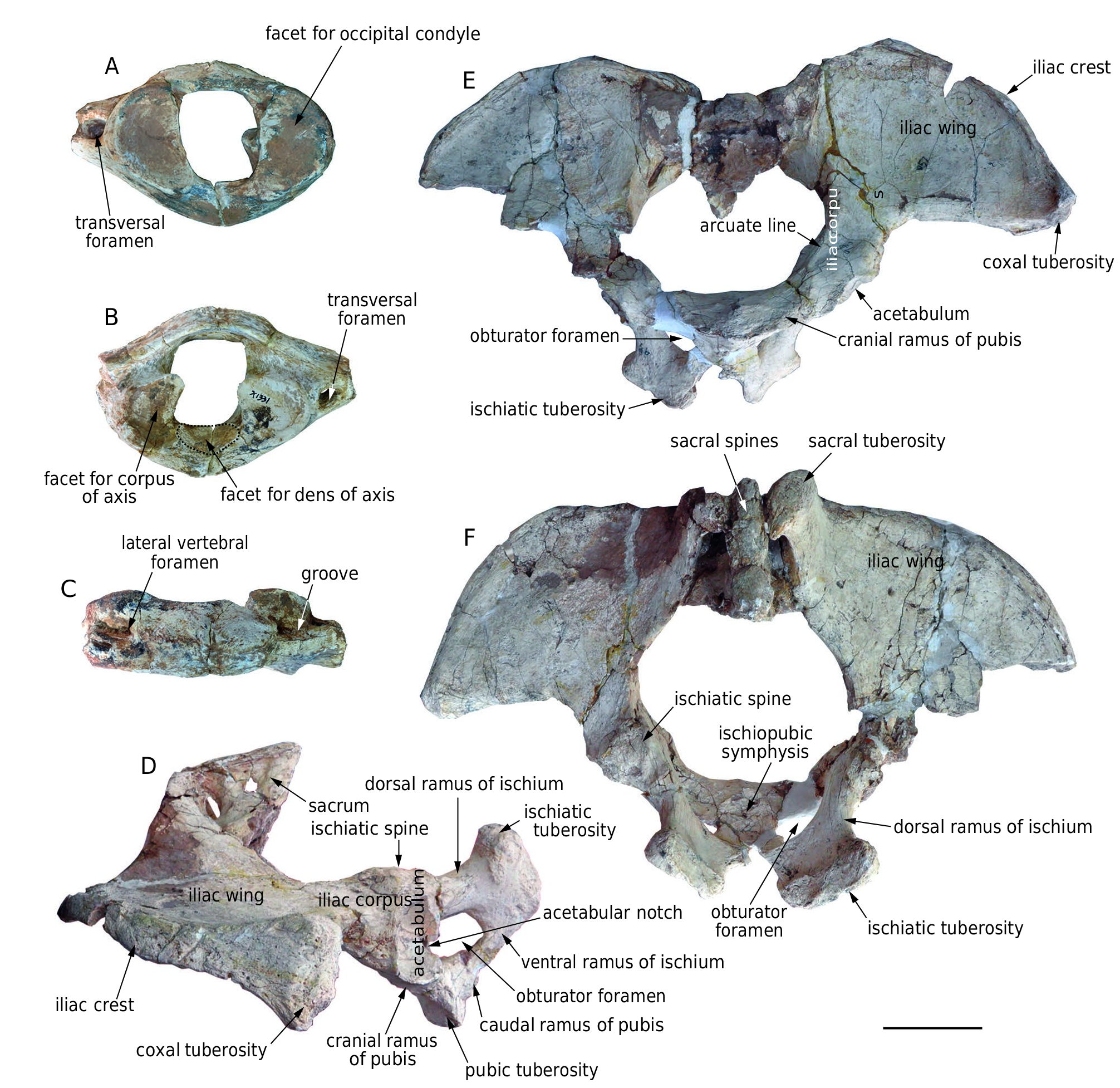
Pelvis ( Fig. 11 View FIG D-F; Table 13). The only pelvis ( IVPP V 18970 View Materials ) is wide with a broad sub-circular aperture. There are partial remains of the sacrum with low and fused spines. The iliac wing is broad and fan-shaped; it is strongly laterally expanded. The sacral tuberosity is thin, upwardly deflected, and turns caudally with a hook-like end. The coxal tuberosity is very thick. The arcuate line is moderately raised in cranioventral view. In lateral view, the pubis, the ischium, and the corpus of the ilium are anteroposteriorly short. The acetabular fossa is oval and its longitudinal axis is almost perpendicular to the main extension of the pelvis. The acetabular fossa is surrounded by a sharp rim with a deep acetabular notch at the middle of the caudal margin. The obturator foramen is sub-circular with the longitudinal axis running almost perpendicular to the acetabular fossa. The cranial branch of the pubis is strong and the caudal branch is thin. The pubic is tightly fused with the other half of the pelvis at the medial side and the pubic tuberosity is thick. The ischium is strongly expanded dorsocaudally in lateral
Specimen IVPP V 18970 View Materials Maximal width between the two coxal tuberosities 355 × 2 Maximal length 409 Width of the pelvic aperture 222 Distance between the sacrum and the cranial
205
end of the symphysis
Distance between the cranial end
of the symphysis and the ventralmost point 274.5
of the sacral tuberosity
Distance between the coxal and sacral
c. 425 tuberosities
Minimal distance between the coxal tuberosity
262
and pelvic aperture
Minimal distance between the coxal tuberosity
218
and acetabulum
Width between two acetabuli 337 Width between two ischiatic spine 233 Symphyseal length 155 Width between two ischiatic tuberosities 214 Minimal width of the iliac corpus 75 Minimal perimeter of the iliac corpus c. 230 Minimal perimeter of the cranial ramus of pubis c. 140 Minimal height of the dorsal ramus of ischium 44 Minimal perimeter of the dorsal ramus of ischium c. 120 Maximal diameter of the acetabulum 77.5 Maximal diameter of the obturator foramen 74
view and strongly extended laterocaudally in caudal view. The cranial branch of the ischium is strong and the caudal branch is thin. The ischiatic tuberosity is very stout and extends craniolaterally-caudomedially.
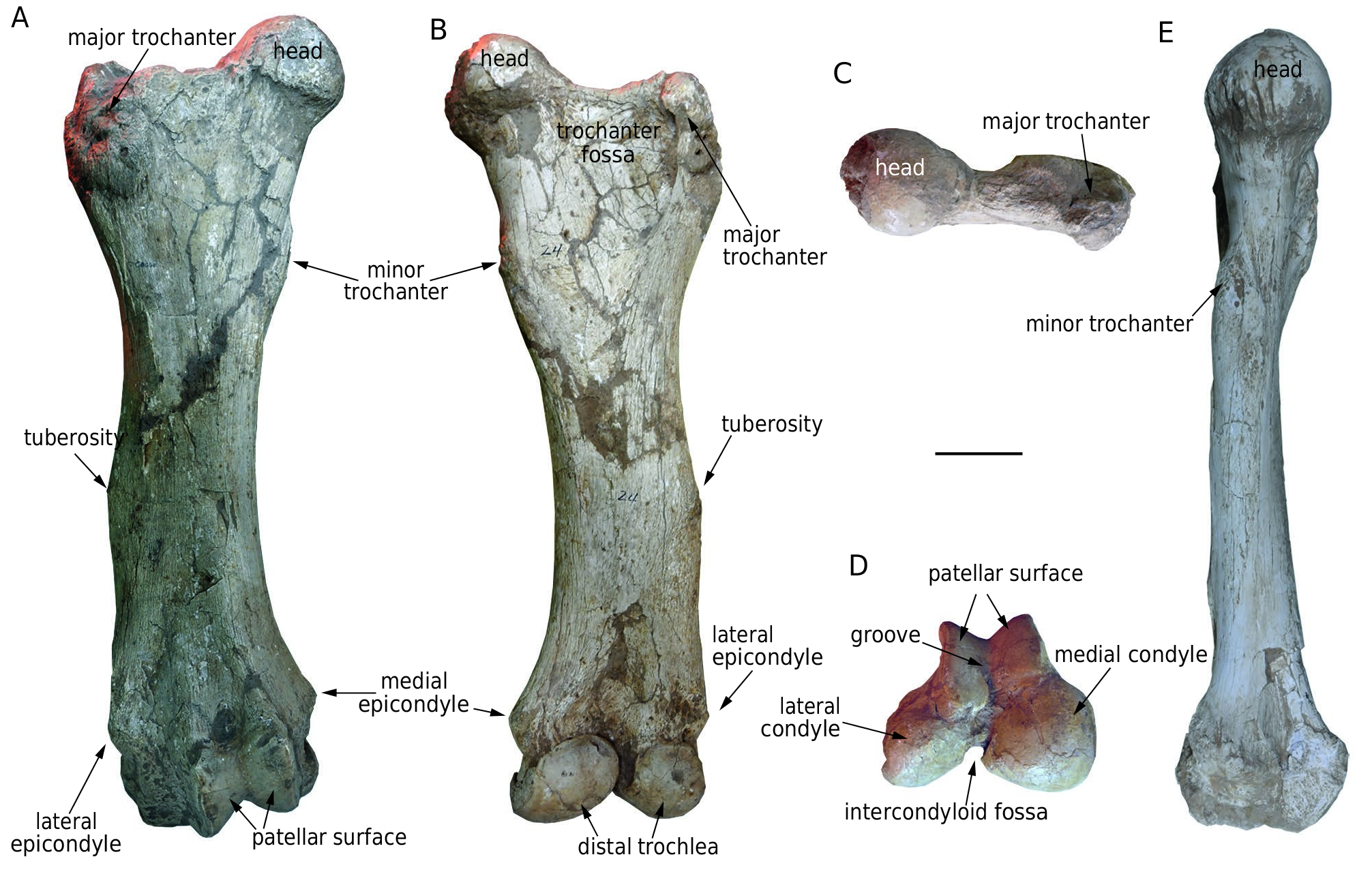
Femur ( Fig. 15 View FIG A-E; Table 14). There are four femora [ HMV 1883 (l.), 0013 (l.), 0002 (l.), and 1897 (r.)] in the study material. The femur is thick. The shaft is long and cylindrical with some craniocaudal compression. The distal two-third of the shaft is slightly convex laterally in cranial or caudal view. The minor trochanter is weak and on the proximal quarter of the medial side of the shaft. On the half of the lateral side of the shaft, a rough and raised tuberosity seems to be for the attachment of the superficial gluteal muscles, the homologue of the third trochanter in perissodactyls. The shaft is enlarged at both extremities. Proximally, the femoral head is markedly hemispheroidal, but relatively small. A rough crest extends laterally from the head and is connected to the major trochanter. The major trochanter is very robust, and more ventrally positioned than the head. It is expanded craniocaudally, and encloses a large, triangular trochanter fossa. Distally, the depressions between the condyles and epicondyles of both sides are deep. The medial and lateral epicondyles are at almost the same level. The distal surfaces are subdivided into a narrow cranial patellar surface and a wide caudal trochlea. The patellar surface is subdivided by a wide, V-shaped valley, and dominated by the medial part. Both parts are oblique craniolaterallycaudomedially. The medial condyle of the distal trochlea is relatively quadrate and larger than the triangular, laterally extended lateral condyle. The two condyles are separated by a deep intercondyloid fossa.
TABLE 14. — Femoral measurements (in mm) of Konobelodon robustus n. sp.
Comparisons. We will compare the postcranial bones of K. robustus n. sp. with those of other gomphotheres using published data, including Gomphotherium sylvaticum Tassy, 1985 (data from Tassy 1977), G. aff. steinheimense (Klähn, 1922) (data from Göhlich 1998), Tetralophodon longirostris (data from Mottl 1969), Haplomastodon chimborazi (Proaño, 1922) (data from Ferretti 2010), K. atticus from Pestszentlőrincz (data from Schlesinger 1922) and K. britti (data from Lambert 1990).
In K. robustus n. sp., the sub-rectangular vertebral foramen of the atlas differs from the pear-shaped foramen of G. sylvati- cum; the thin and low dorsal arch is like that of T. longirostris , in contrast to the stout and high arch of H. chimborazi ; the facet for the dens of the axis is larger and more triangular than that of G. sylvaticum and G. aff. steinheimense. In K. robustus n. sp., the iliac crest of the pelvic is not oblique laterocaudally, whereas in G. aff. steinheimense, H. chimborazi and K. atticus , the iliac crest is oblique; the oval acetabular fossa differs from the sub-circular acetabular fossa in K. atticus . The obturator foramen of K. robustus n. sp. is sub-circular with the longitudinal axis running almost perpendicular to the acetabular fossa, very unlike the elongated obturator foramen in K. atticus and in G. aff. steinheimense, which is oblique to the acetabular fossa.
In K. robustus n. sp., the lateral expansion of the humeral lateral condyloid crest is more prominent than that of G. sylvaticum , G. aff. steinheimense, and T. longirostris , but not as prominent as that of H. chimborazi ; the proximal lateral tuberosity is higher than the humeral head, similar to that of H. chimborazi , but distinct from those at almost the same level in G. sylvaticum , G. aff. steinheimense, and T. longirostris . In K. robustus n. sp., the shape of the radial notch is similar to that of G. sylvaticum , and is deeper and narrower than those of G. aff. steinheimense and H. chimborazi . The facets for the radius in the radial notch are separated, on both the dorsomedial and the dorsolateral sides, in contrast with the single, not separated facet in G. aff. steinheimense and G. sylvaticum . Like the radius, the proximal facet for the ulna is separated in K. robustus n. sp.; and singular in G. aff. steinheimense and G. sylvaticum . The slight lateral convexity of the distal two-thirds of the femoral shaft in cranial or caudal view in K. robustus n. sp. is also visible in T. longirostris .
The limb bones of K. robustus n. sp. are very robust ( Fig. 16). Although relatively short, the humerus, ulna, and femur are thicker than those of known gomphotheres (except the humerus of Gomphotherium productum (Cope, 1874) , which is almost the same thickness as that of K. robustus n. sp.), amebelodontines, and far thicker than those of extant elephants (every vertical coordinate in Fig. 16). The measurements indicate that the femur of K. robustus n. sp. is much stouter than that of K. britti ( Lambert 1990) . Only the metacarpal IV is thinner than that of the South American Haplomastodon chimborazi , the extremities of which are very specialized ( Fig. 16). However, the metacarpal IV is still thicker than in other gomphotheres and extant elephants. The humerus of K. robustus n. sp. is relatively short; it is as long as the ulna, and much shorter than the femur ( Fig. 16). However, in extant elephants and some brevirostrine gomphotheres (such as Haplomastodon chimborazi ), the humerus is longer than the ulna, and is close to the length of the femur ( Fig. 16). Gomphotheres generally have thicker long bones and a shorter humerus than extant elephants, which has been previously noted, but they do not have as large a body mass as extant elephants ( Christiansen 2004). This can be interpreted as resulting from a more columnar standing posture in extant elephants ( Christiansen 2007). We estimated the body mass of K. robustus n. sp. based on the dimensions of the long bones ( Christiansen 2004). The body mass is in the range 2802- 7367 kg ( Table 15), which is generally larger than that of Archaeobelodon filholi (2985-3477 kg), Gomphotherium angustidens (2956-3980 kg), and G. productum (2304-5429 kg), but smaller than Cuvieronius hyodon (2994-7753 kg), Stegomastodon platensis (4336-7260 kg) and various true elephantids ( Christiansen 2004). Therefore, on the one hand, K. robustus n. sp. has a relatively larger body mass than other gomphotheres (except the American brevirostrine gomphotheres), as the limb bones are thicker; on the other hand, its standing posture may not have been as columnlike as that of extant elephants and American brevirostrine gomphotheres.
COMPARISON WITH AMEBELODON
Since Konobelodon was initially established as a subgenus of Amebelodon ( Lambert, 1990) , here comparisons between K. robustus n. sp. and Amebelodon are further emphasized. Five species have been included in Amebelodon , i.e. A. fricki , A. floridanus (Leidy, 1886) , A. hicksi (Cook, 1922) , A. paladentatus (Cook, 1922) , and A. sinclairi Barbour, 1930 ( Lambert, 1990) . All of the species are represented by mandibular and/ or dental material, and the cranial anatomy of Amebelodon is virtually unknown ( Lambert 1996). Because Konobelodon has been removed from Amebelodon , the diagnosis of Amebelodon should be confined to the subgenus Amebelodon (Amebelodon) of Lambert (1990), i.e. trilophodont intermediate cheek teeth; lower tusks with simple laminated internal structure; M3/m3 with five or fewer loph(id)s. All characters are distinct from K. robustus n. sp.
A lateral enamel band is present on the upper tusks of Amebelodon . This band appears to be absent in K. robustus n. sp., although upper tusks of adult individuals of K. robustus n. sp. are unknown. In juvenile individuals of K. robustus n. sp., the anterior part of the upper tusks possesses an enamel cap that never extends posterolaterally ( Fig. 8A, B View FIG ).
Lower tusks of Amebelodon are narrow, elongated, and flattened with a shallow dorsal concavity, which is similar to those of K. robustus n. sp.. However, other features of the lower tusks in Amebelodon are notably distinct from those of K. robustus n. sp. In Amebelodon , besides the absence of internal dentinal tubules, the lower tusks are characterized by a gradually smooth and polished anterior tip without distinct medial and lateral angles; the exposed length of the lower tusks are not longer than the symphyseal length in adult individuals; and the two tusks are convergent ( Fig. 5A View FIG ).
Apart from the number of loph(id)s, the morphology of the cheek teeth in Amebelodon and K. robustus n. sp. are also different. In the cheek teeth of Amebelodon , posttrite trefoils and pseudo-anancoidy are strong, showing a complicated pattern. However, in K. robustus n. sp., the posttrite trefoils and pseudo-anancoidy are incipient, the cheek teeth show more “lophodont” features ( Fig. 10 View FIG ).
Despite the differences of dental feature within the two group, the mandibular morphology of Amebelodon and K. robustus n. sp. resembles with each other. In dorsal view, the mandibular symphysis of Amebelodon only slightly expand laterally in the distal part, similar to the not laterally expanded symphysis of K. robustus n. sp. ( Fig. 5A, F View FIG ). In lateral view, the mandibular symphysis of Amebelodon and K. robustus n. sp. is downward deflected almost in the same angle ( Fig. 5M, N View FIG ). The mandibular ramus of both Amebelodon and K. robustus n. sp. is almost perpendicular to the occlusal plan, indicating a similar distribution of the jaw-closing mucles, which possibly represents similar feeding behavior.
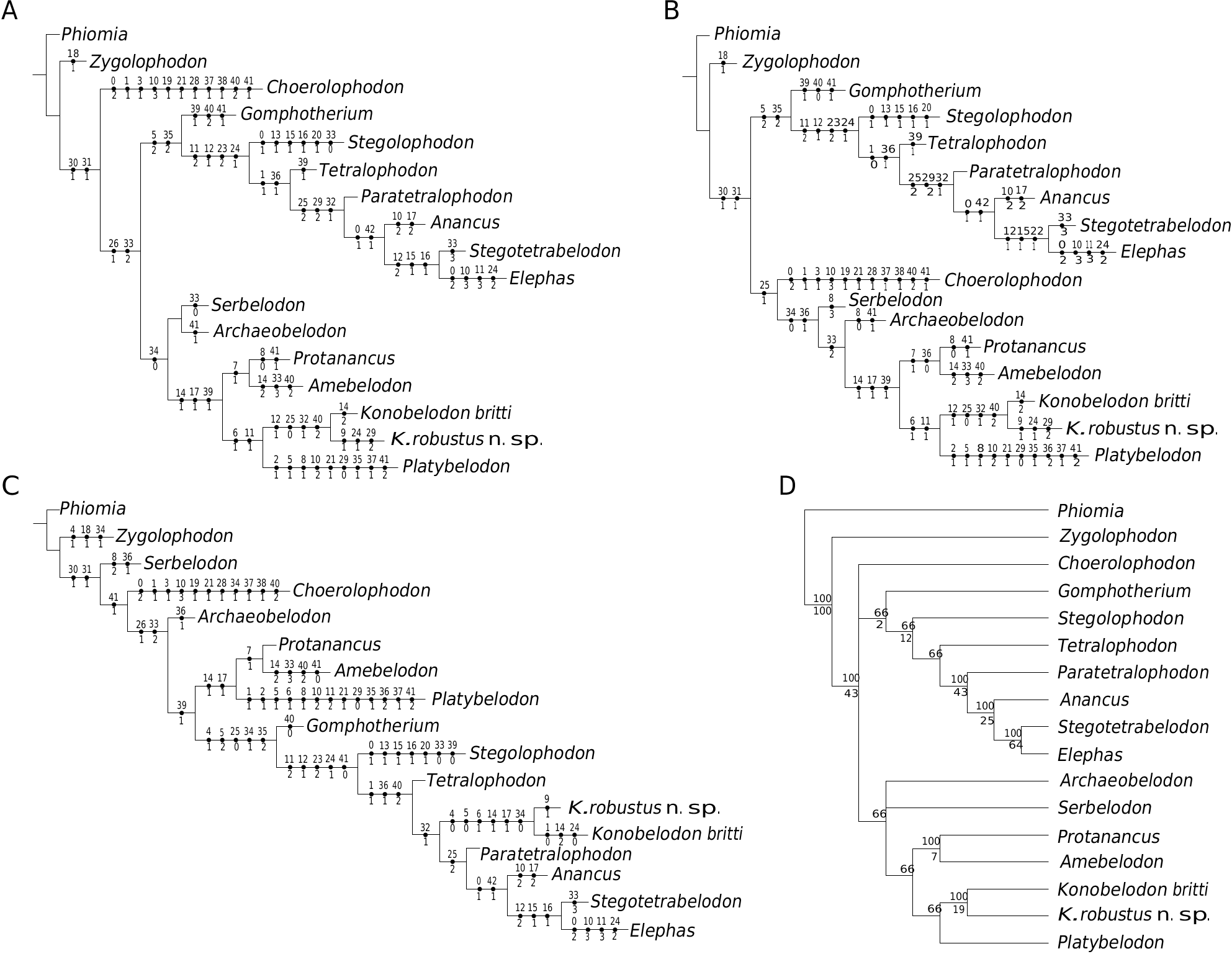
CLADISTIC ANALYSIS
The cladistic analyses of Kalb et al. (1996) and Ferretti et al. (2003) showed that “ M. grandincisivus ” is the sister group of tetralophodont gomphotheres, elephantids, and stegodontids, but is not clustered with the stegotetrabelodontines.However, those analyses did not include the amebelodontines. In our cladistic analysis, we incorporated members of amebelodontines in the dataset. Three MPTs were obtained (tree length = 116, consistency index = 0.586, retention index = 0.625, Fig. 17 View FIG ). As we expected, in all three MPTs K. robustus n. sp. clusters with K. britti , supporting our generic attribution. In one MPT ( Fig. 17C View FIG ), Konobelodon makes up the sister group of Paratetralophodon , Anancus and other elephantids, and both groups are derived from the Gomphotherium stock with other tetralophodont gomphotheres ( Tetralophodon and Stegolophodon ). Supporting characters include 11, 12, 23, 24, and 41. This relationship can not be excluded, because the teeth of K. robustus n. sp. and K. atticus are more similar to those of Tetralophodon rather than amebelodontines (however, the shared cheek teeth characters in K. robustus n. sp., K. atticus , and Tetralophodon are plesiomorphies thus not suitable for grouping taxa). Furthermore, in this MPT, other amebelodontines do not cluster either, and constitute a paraphyletic group; after all, the flattened lower tusk is also a plesiomorphy shared with the outgroup Phiomia .
In the other two MPTs, all members of the amebelodontines are clustered, forming a monophyletic group ( Fig. 17A, B View FIG ). As an amebelodontine, Konobelodon makes up the sister group of Platybelodon , supported by characters 6 and 11. These results are more likely to be accepted by most researchers. In K. robustus n. sp., the neurocranium is arched and the basicranium is erected, as in derived gomphotheres and elephantids. However, this process seems to have been evolved more than once in different proboscidean clades, and thus possibly represents parallel evolution rather than a true synapomorphy. Tassy (1986, 1999) suggested that “ M. grandincisivus ” is related to Platybelodon and Konidaris et al. (2014) proposed that Konobelodon could have derived from a Platybelodon stock. However, we still question the sister-group relationships of Konobelodon and Platybelodon . Platybelodon is a very specialized group within amebelodontines. The horizontal succession of the cheek teeth is notably progressed, and the limb bones of Platybelodon are very slender ( Fig. 16; also see Wang & Ye 2015), markedly unlike the robust limb bones in K. robustus n. sp. The tetralophodont m2 of the two taxa are distinct, as discussed above. The only strong supporting feature is the presence of the tubular structure.Evidence shows that a lower tusk with tubular structure has mechanical advantages for higher load and abrasion (Wang et al. 2015), thus this structure may have been independently derived as a result of environmental selection pressure. In our hypothesis, Konobelodon is possibly more closely related to Amebelodon rather than to Platybelodon , as it was originally established as a subgenus of Amebelodon , because the similarity of mandibles of Konobelodon and Amebelodon ; however, more evidence is required for this hypothesis. Herein we also provide a 50% majority rule tree ( Fig. 17D View FIG ). This is an acceptable result at the present stage.
RECONSTRUCTION OF THE SKULL
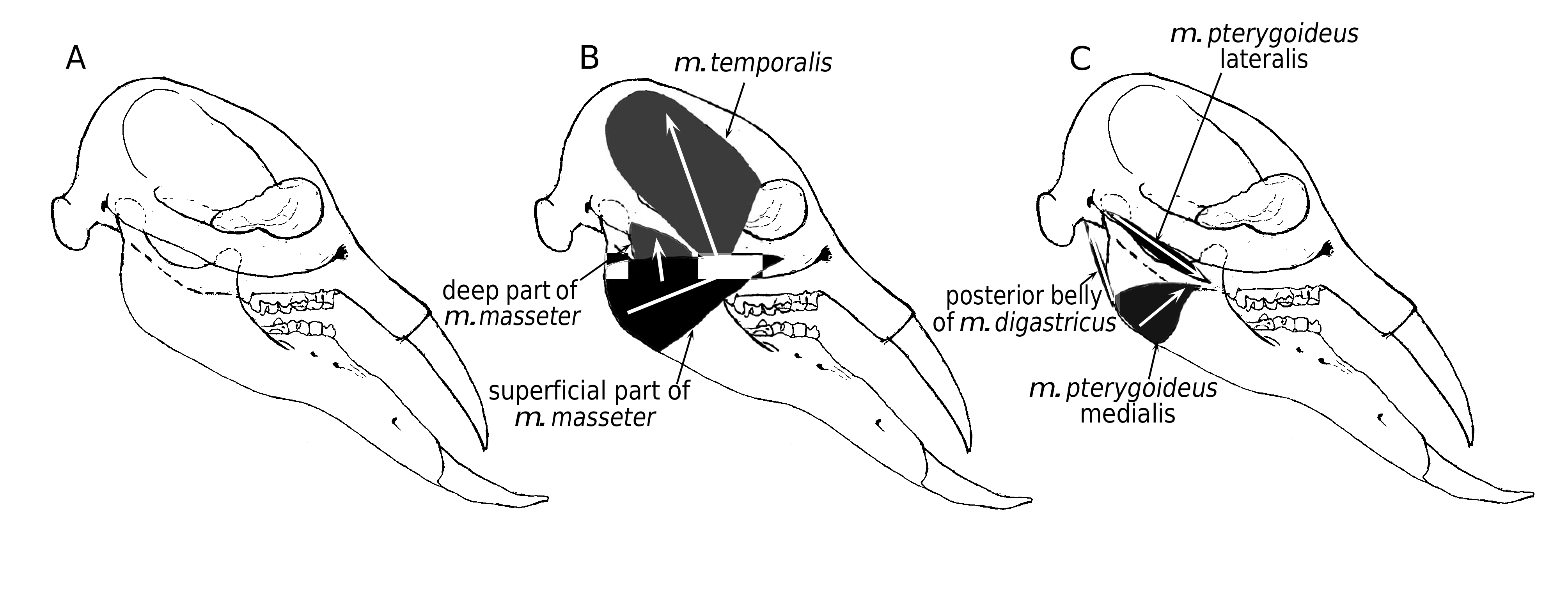
We reconstructed the juvenile skull of Konobelodon robustus n. sp. based on the paratypes HMV 1904 and 1910 ( Fig. 18A View FIG ) and also reconstructed the jaw-closing muscles (see Maglio 1972; Ye et al. 1990; Tassy 2014) ( Fig. 18B, C View FIG ). The tip of the upper tusk does not anteriorly surpass the mandibular symphysis and the facial part is not anteriorly elongated. More importantly, the ascending ramus is vertical, unlike the posteriorly oblique ramus in Platybelodon grangeri and Gomphotherium angustidens . Therefore the composite force of the m. temporalis and the deep part of the m. masseter is relatively perpendicular to the occlusal surface ( Fig. 18B View FIG ); however, in Platybelodon grangeri the same composite force is severely posteriorly oblique. In the latter case, a small moment produced on the mandibular condyle (taken as the pivot of mandibular movements) is resultant and the force component perpendicular to the main extension of the mandible is also relatively small. Therefore, the mandible and lower tusks of Platybelodon grangeri are specialized and more suitable for cutting soft vegetation (the main external force is nearly along the main extension of the mandible) than for digging on hard substrate (the main external force is not along the main extension of the mandible but has a considerable perpendicular component). This finding supports the similar conclusion of Lambert (1992) based on observations of the wear facets and microwear on the lower tusks. The above discussion is only a preliminary qualitative analysis. Further studies should be carried out to better interpret the relationship between feeding behaviors and mandibular morphology of amebelodontines, especially quantitative studies such as finite element methods. Conversely, in elephantids, the lower tusks have been completely lost, and the ascending ramus is anteriorly inclined, yielding a composite force nearly perpendicular to the occlusal surface from the temporal muscle.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Konobelodon robustus
| Wang, ShiQi, Shi, QinQin, He, Wen, Chen, ShanQin & Yang, XiangWen 2016 |
Tetralophodon
| DENG T. & QIU Z. - X. & WANG B. - Y. & WANG X. - M. & HOU S. - K. 2013: 256 |
| DENG T. & WANG X. - M. & NI X. - J. & LIU L. - P. 2004: 11 |
Tetralophodon exoletus
| DENG T. & QIU Z. - X. & WANG B. - Y. & WANG X. - M. & HOU S. - K. 2013: 257 |
| DENG T. & WANG X. - M. & NI X. - J. & LIU L. - P. 2004: 11 |