CALLOPORIDAE Norman, 1903
|
publication ID |
https://doi.org/ 10.1080/00222930500415195 |
|
persistent identifier |
https://treatment.plazi.org/id/03CE7B54-FFD6-FFD5-DE99-1E388901B8A2 |
|
treatment provided by |
Felipe |
|
scientific name |
CALLOPORIDAE Norman, 1903 |
| status |
|
Family CALLOPORIDAE Norman, 1903 View in CoL
Genus Callopora Gray, 1848 View in CoL Callopora craticula ( Alder, 1856) View in CoL
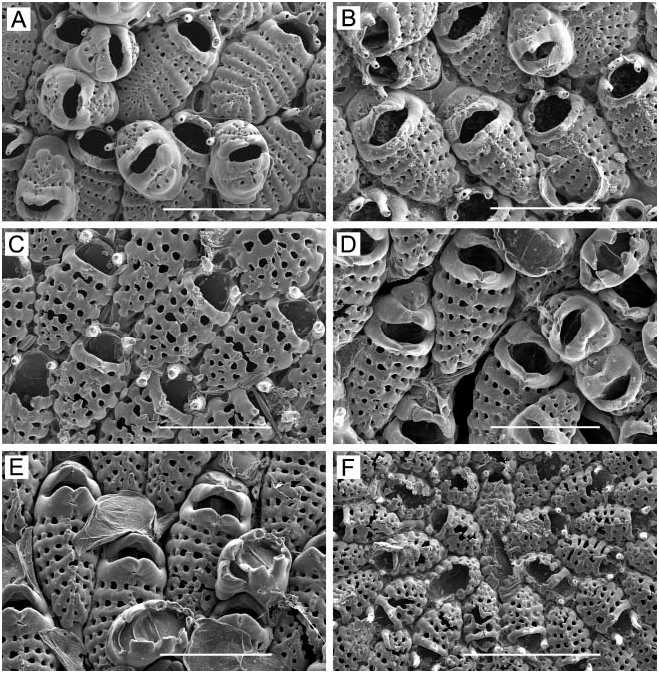
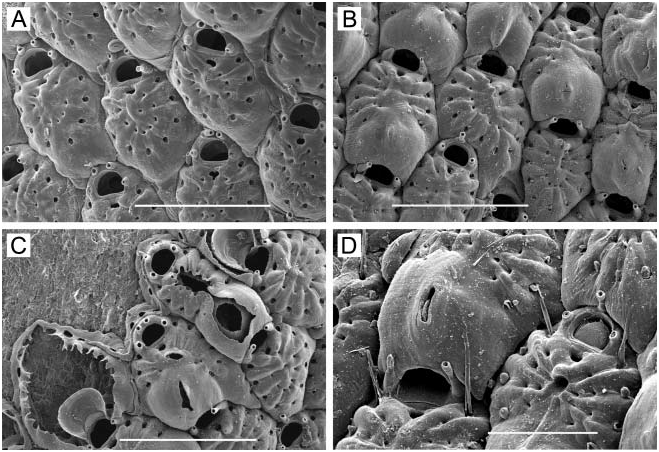
( Figure 2A–D)
Membranipora craticula Alder 1856, p 144 .
Callopora craticula: Osburn 1950, p 67 View in CoL , Plate 6, Figure 7 View Figure 7 ; Kluge 1975, p 344, Figure 171; Mawatari and Mawatari 1980, p 40, Figure 8 View Figure 8 ; Dick and Ross 1986, p 89 (in part); 1988, p 33, Plate 1C.
Description
Colony. Unilaminar, encrusting, sheet-like, whitish to tan in colour; delicate, largest colony observed 4 mm across.
Zooids. Small, 0.30–0.43 mm long (average 50.357 mm, n 515, 3) by 0.16–0.26 mm wide (average 50.218 mm, n 515, 3); barrel-shaped to oval, proximal end truncate or tapering between adjacent zooids; separated by a groove. Lateral and proximal gymnocyst smooth, sloping, extensive; proximal gymnocyst comprising one-third to two-fifths of zooid length. Cryptocyst negligible, a narrow, smooth, sloping shelf inside mural rim. Opesia oval, often widest proximally, 0.16–0.25 mm long (average 50.190 mm, n 515, 3) by 0.10–0.14 mm wide (average 50.120 mm, n 515, 3).
Spines. Twelve to 17, arranged as three or four pairs of long, heavy orificial spines, truncate and open at the end, erect or angled distally, sometimes curved laterally; and 7–10 finer, acute opesial spines around proximal half of opesia, angled inward, the tips meeting in the midline ( Figure 2A, B); all spines tubular, not flattened. The distalmost pair of opesial spines is intermediate in character, long and open at the end like the orificial spines, but tapering, subacute, the tips meeting in the midline like the more proximal acute spines. In ovicellate zooids, the distalmost pair of orificial spines is often embedded in the lateral ovicell wall on one or both sides; the second pair often abuts or is embedded in the proximal corners of the ovicell ( Figure 2C).
Avicularia. Zooids typically have a frontal avicularium with a raised chamber on the gymnocyst close to the proximal opesial margin, the acute, long-triangular mandible pointing proximally ( Figure 2A, C); lacking in some zooids; if preceded by an ovicell, this avicularium tends to be larger, the chamber frequently overlapping the distal end of the ovicell, with the mandible pointing distally ( Figure 2C). Occasionally, the avicularium occurs on the lateral rather than the proximal gymnocyst. Vicarious avicularia ( Figure 2A, B) occur sporadically; these are almost as large as autozooids, with the rostrum bearing an acute, long-triangular mandible extending past the distal end of the chamber.
Ovicell ( Figure 2A–C). Raised, hemispherical, imperforate, 0.15–0.16 mm long (average5 0.154 mm, n 510, 2) by 0.15–0.19 mm wide (average 50.161 mm, n 510, 2); with a conspicuous, often decurved transverse ridge across the top marking proximal extent of ectooecium; lumen between ectooecium and endooecium typically filled with calcification in mature zooids ( Figure 2C); proximal margin raised as a sharp vertical lip.
Ancestrula . With nine spines ( Figure 2D), initially budding a daughter zooid distally, then two additional zooids, one on each side from junction between ancestrula and first daughter zooid.
Remarks
Callopora craticula View in CoL formed small colonies growing on rocks and on serpulid worm tubes attached to rocks. Our specimens are similar to most descriptions from other parts of the range. For example, zooids are small, 0.30–0.43 mm at Ketchikan, 0.35–0.45 mm at Kodiak ( Dick and Ross 1988), and 0.30–0.38 mm in Britain ( Hayward and Ryland 1998); range of spine number is 12–17 at Ketchikan, 11–16 in the Russian Far Eastern seas ( Kluge 1975), 12–15 at Kodiak ( Dick and Ross 1988), and 12–15 in Britain ( Hayward and Ryland 1998). However, Osburn (1950) indicated both larger zooids (0.40–0.55 mm) and more spines (14–18) for eastern Pacific specimens, and Mawatari and Mawatari (1980) indicated more spines (10–19 proximal and two to four distal) for specimens from northern Japan; these records of C. craticula View in CoL may represent other species.
We have examined SEM images, kindly sent by Dr Piotr Kuklinski, of two British specimens of C. craticula . One specimen is from the Northumberland Coast, probably a syntype, collected by J. Alder; the other comes from the Norman collection (NHM 1911.10.1.523), labelled as collected by J. Alder from the Durham coast. The spines in these specimens are somewhat more regular than in our material, meaning they are more evenly formed and more regularly angled and tilted to form a neater basket over the opesia. However, the overall arrangement of spines is identical, with acuminate spines meeting in the midline over the proximal half of the opesia; two to four pairs of long, blunt, erect or distally angled spines, with those immediately lateral to the orifice often curved outward; and the distalmost pair of opesial spines of mixed character between the orificial and other opesial spines. As in our material, the distalmost pair of spines is often embedded in the lateral walls of the ovicell .
We can find no diagnostic differences in other characters between Alder’s material and ours, such as in the form, size, distribution, and direction of frontal avicularia; form of the gymnocyst and cryptocyst; and form of the ovicell. SEM images of a specimen from Spitsbergen, provided by Dr Piotr Kuklinski, show a similar ancestrula: tatiform with nine spines, initially giving rise to three zooids distally. A questionable character for C. craticula has been the occurrence of vicarious avicularia. Although Kluge (1975) and Dick and Ross 1988) reported them, neither Hincks (1880b) nor Hayward and Ryland (1998) mentioned them for C. craticula from Britain. However, Hincks (1880b, Plate 19, Figure 7 View Figure 7 ) showed one in his illustration, and a vicarious avicularium is present in the Durham specimen mentioned above (NHM 1911.10.1.523). Therefore, the presence of vicarious avicularia must be regarded as a character of C. craticula .
Distribution
This is a circumpolar, arctic-boreal species; Kluge (1975) summarizes many previous records. C. craticula has been reported as far south as Scotland and northern England in Britain ( Hayward and Ryland 1998), Wood’s Hole in the western Atlantic ( Osburn 1912; but see Winston et al. 2000), and southern Hokkaido in Japan ( Mawatari and Mawatari 1980). In the eastern Pacific, Ketchikan is a little farther south than the previous southernmost record at Frederick Sound ( Osburn 1950).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
CALLOPORIDAE Norman, 1903
| Dick, Matthew H., Grischenko, Andrei V. & Mawatari, Shunsuke F. 2005 |
Callopora craticula:
| Dick MH & Ross JRP 1986: 89 |
| Mawatari S & Mawatari SF 1980: 40 |
| Kluge GA 1975: 344 |
| Osburn RC 1950: 67 |
Membranipora craticula
| Alder J 1856: 144 |