Bosmina (Bosmina), (BOSMINA)
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2008.00475.x |
|
publication LSID |
lsid:zoobank.org:pub:32BB4BC4-3FE1-4ED8-AE9D-D753AB54EDFC |
|
persistent identifier |
https://treatment.plazi.org/id/03CC87C6-FFCD-FFDB-471B-FC9ECF6CDF40 |
|
treatment provided by |
Felipe |
|
scientific name |
Bosmina (Bosmina) |
| status |
|
SUBGENUS BOSMINA (BOSMINA) View in CoL BAIRD, 1845
Eunica Koch, 1841: 23 View in CoL (preoccupied name, Huebner, 1819; Lepidoptera View in CoL ).
Bosmina Baird, 1845: 149 View in CoL ; Baird, 1846: 412.
Bosmina (Bosmina) View in CoL in Lieder, 1957 (manuscript); Lieder, 1962: 317; Lieder, 1983b: 123, 126; De Melo & Hebert, 1994: 1818; Taylor et al., 2002: 1494.
Bosmina (Sinobosmina) in De Melo & Hebert, 1994: 1820 View in CoL .
Garbinia Grochowski, 1910: 343 .
Type species: Monoculus cornutus Jurine, 1820. When Baird (1845) established the genus Bosmina , it was monotypical, containing only a single species Bosmina cornuta ( Jurine, 1820) . At the same time, the author listed ‘ Lynceus longirostris ? Muller’ as a possible synonym of B. cornuta . Now, B. cornuta is regarded as a junior synonym of B. longirostris .
Subgenus diagnosis based on male characters: Distal portion of postabdomen as a tube, not inflated, preanal margin more or less depressed, with relatively long, fine setules. Gonopore opens distally. Postabdominal claw with a sharp terminal spinule. Basal pecten of denticles shifted from postabdominal claw to body of postabdomen, distal pecten consisting of short, robust denticles. Antenna I with relatively thin preaesthetasc portion. Copulatory hook on limb I strongly narrowing distally. On subdistal lobe of limb I, all setae located closely. Seta 2 on limb-I corm, not very short.
Comment: Numerous species, forms, and varieties of B. cf. longirostris described from Europe (see Lieder, 1996: 33–34), and from some other areas, were established on characters, such as the curvature of the female antennule, that are subject to significant intra- and interpopulation variability. In some German lakes the morphs coexist, and show overlapping but significant morphological differentiation ( Kappes & Sinsch, 2002a, b). These have been regarded as separate species ( B. cornuta and Bosmina pellucida Stingelin, 1895 ) by Kappes & Sinsch (2002a, b, 2005), but it is unknown if these morphs represent spatial polymorphisms, simple morphological polymorphisms, or cryptic invasive species. We lacked genetic evidence for more than one species of B. cf. longirostris in Europe – further studies are needed to assess the species diversity of European longirostris -like morphotypes.
At the same time, several other species from the subgenus B. ( Bosmina ) have been discovered in North America ( De Melo & Hebert, 1994) and Asia ( Kořínek, Saha & Bhattacharya, 1999), although they were initially incorrectly placed in the subgenus Sinobosmina . Recent molecular data ( Taylor et al., 2002) and the present analysis on males confirmed the position of B. liederi and B. freyi in the subgenus Bosmina s.s. Moreover, our morphological analysis (see cladogram) indicates that Bosmina tripurae Kořínek, Saha & Bhattacharya, 1999 is also a member of Bosmina s.s. Previous authors ( Lieder, 1983b; De Melo & Hebert, 1994; Kořínek et al., 1999) assigned species to the subgenus Sinobosmina when the lateral head pore was located near the edge of the head shield. But, the extreme lateral position of the head pore is a peculiarity of B. longirostris only, and B. tripurae has a pore located at a small distance from the edge of the head shield, as in the Sinobosmina . Combined genetic and morphological analyses of more Sinobosmina and Palearctic Bosmina specimens are necessary to further test the reliability of the lhp position for separating Sinobosmina and Bosmina .
Unfortunately, we have no males of B. freyi . In addition, there are some other undescribed species in North America ( Little et al., 1997; Kim et al., 2006). So, our investigation is only a first step in the revision of the subgenus in North America.
Bosmina (Bosmina) longirostris (O. F. Müller, 1776) View in CoL , Figures 1–4 View Figure 1 View Figure 2 View Figure 3 View Figure 4
Lynceus longirostris O. F. Müller, 1776: 199 ; O. F. Müller, 1785: 76–77; pl. 10, figs 7, 8.
Bosmina longirostris (O. F. Müller) View in CoL in Baird 1850: 105–106; P. E. Müller, 1867: 146; pl. 3, figs 8, 9; Lilljeborg, 1901: 225–236; pl. 30, figs 13–16; pl. 31, figs 1–18; pl. 32, figs 1–3; Keilhack, 1908: 444–445; figs 4–7; Uéno, 1927: 285–287; pl. 26, fig. 15a–f; Burckhardt, 1941: 130–141; figs 6, 7, 10, 11, 17, 25, 26, 28; Šrámek-Hušek, Strašcraba & Brtek, 1962: 277–280; fig. 101; Margaritora, 1983: 32–34; fig. 18A– G; Margaritora, 1985: 56–60; figs 25, 26; Sars, 1993: 79–80; pl. 61; Alonso, 1996: 248–251; figs 111–112.
Bosmina (Bosmina) longirostris (O. F. Müller) View in CoL in Flössner, 1972: 214–217; figs 100, 101; Lieder, 1983b: 126; figs 1, 7a, 8a; Negrea, 1983: 219–225; figs 88–90. Bosmina longirostris-curvirostris Fischer in Keilhack, 1909: 52; figs 127, 128.
Garbinia adriani Grochowski, 1910: 343 View in CoL , text – figs a–b.
? Bosmina pellucida Stingelin, 1895: 229 View in CoL , figs 22, 23. Not Bosmina (Bosmina) longirostris View in CoL in Chiang Siehchin & Du Nan-shan, 1979: fig. 110C; De Melo & Hebert, 1994: 1818–1819; fig. 10.
Material (males): Belgium. Duck pond near Ghent University, collected on 21 October 1997 by K. Van Damme and 5 November 1997 by A. A. Kotov, AAK 2002-177 .
Germany. Globsow See , Berlin, collected on 27 October 1998 by M. Abashanin, AAK 2004–027 .
Norway. A bog lake near Oslo, collected on 7 September 1990 by N. N. Smirnov, AAK 2004–028 .
Russia (European). Lake Glubokoe, Ruza District , Moscow Area , collected on 1 December 1996 by A. A. Kotov, AAK 2004–043 ; Sterlazhij Pond, Zvenigorod District, Moscow Area , collected on 10 October 1999 by A. A. Kotov, AAK 2004–008 ; Istra Water Reservoir, Moscow Area , collected on 30 June 1980 by N. N. Smirnov, AAK 2004–025 . A pond near main office of the Teberda Nature Reserve, Karachayevo-Cherkess Autonomous Republic , collected on 1 July 1980 by V . Spiridonov , AAK 2004–022 .
Iraq. Zafaraniya , collected on 28 January 1974 by N. N. Smirnov, AAK 2004–034 .
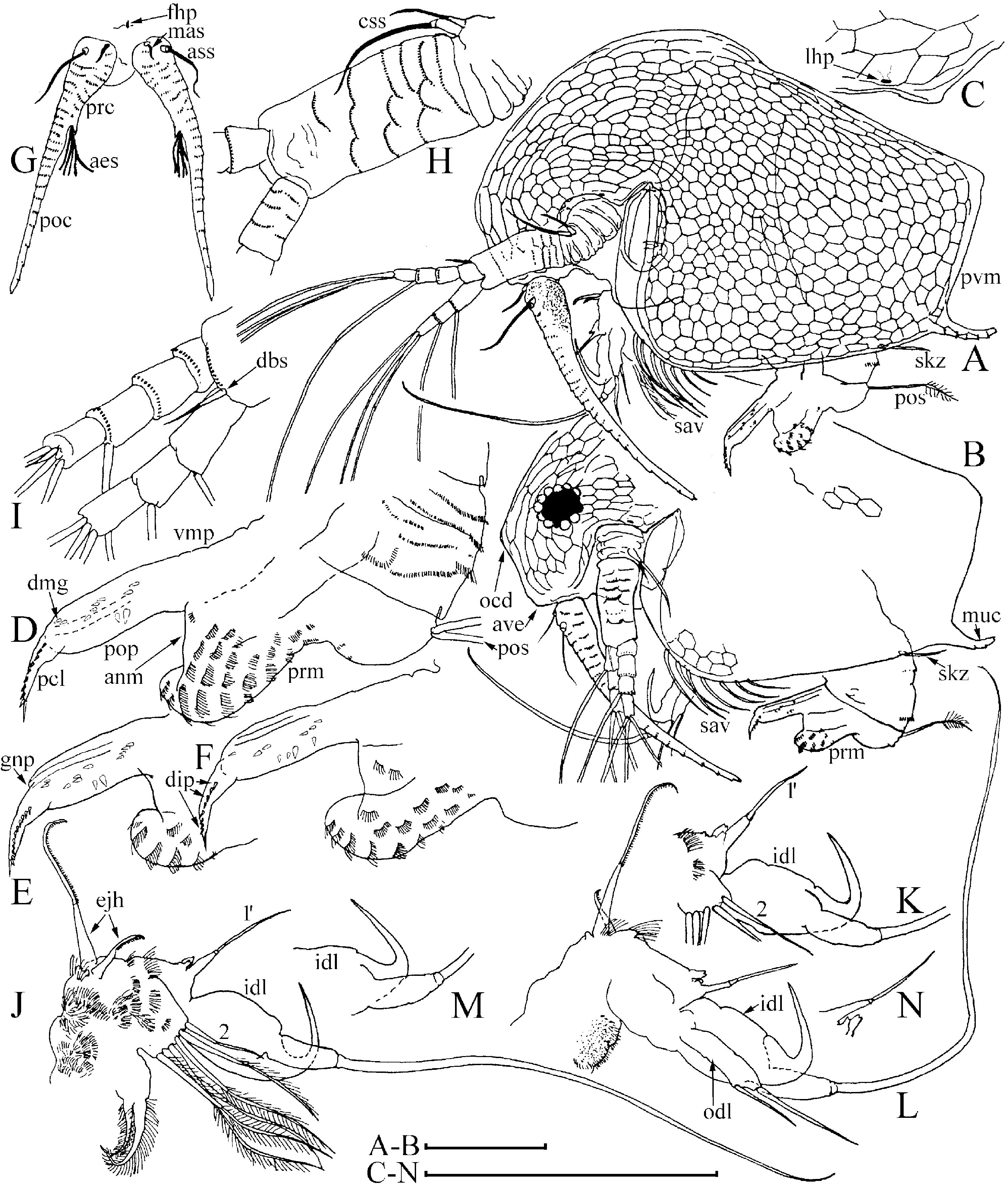
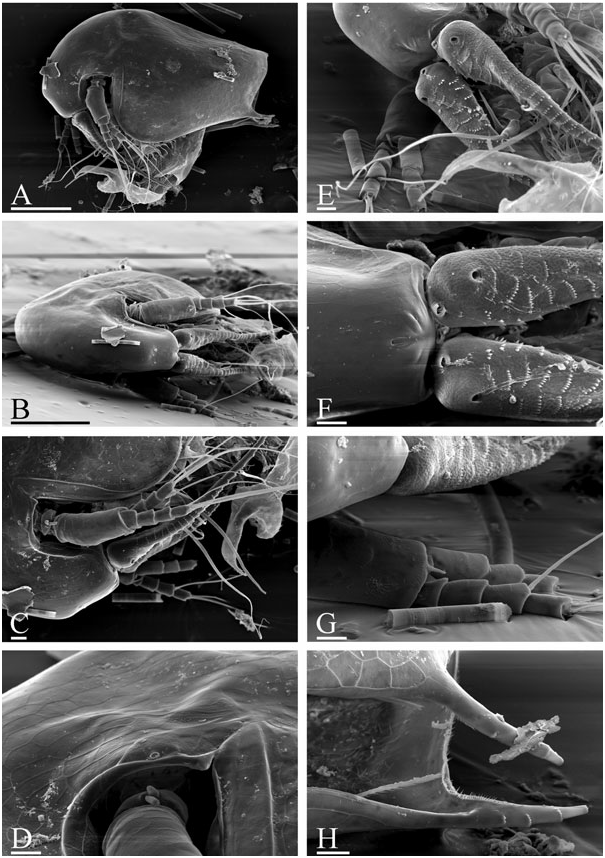
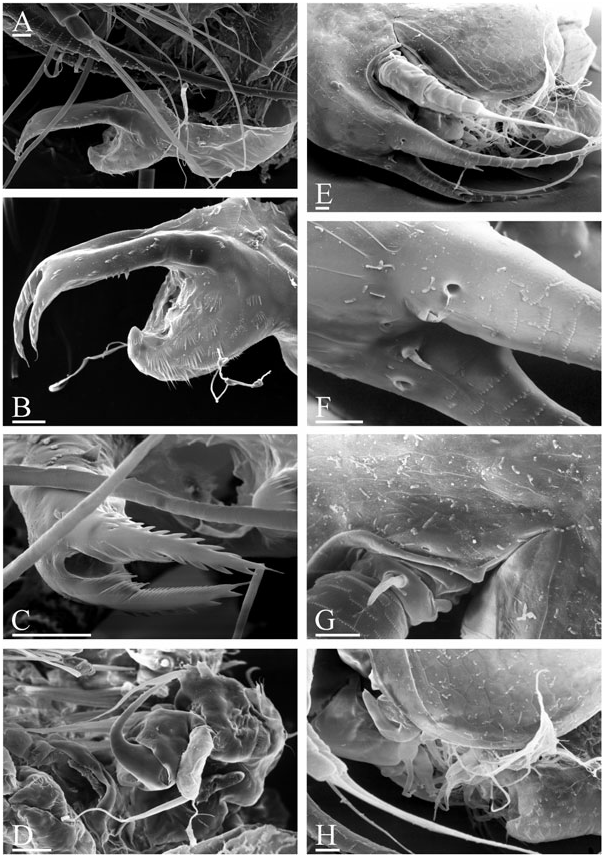
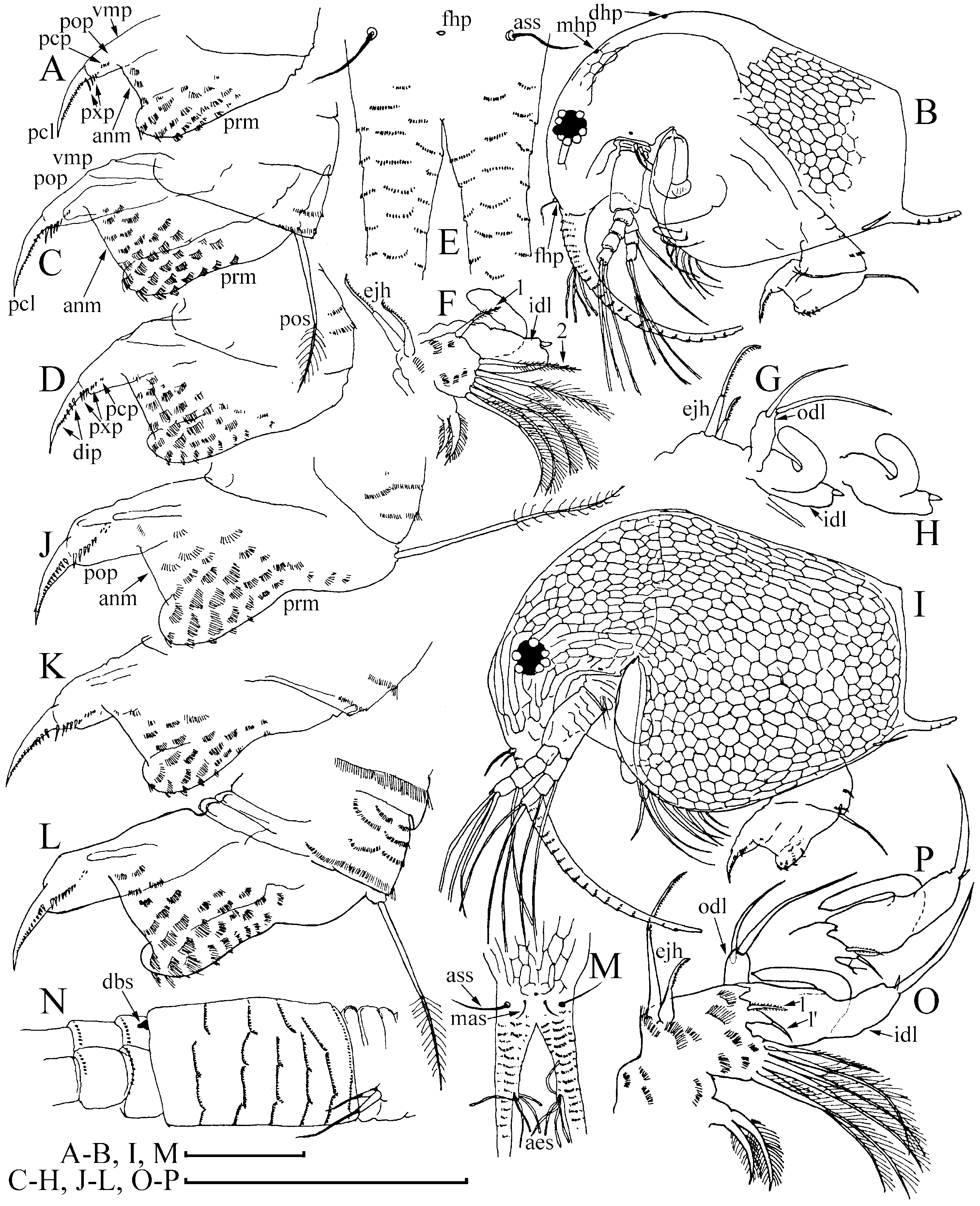
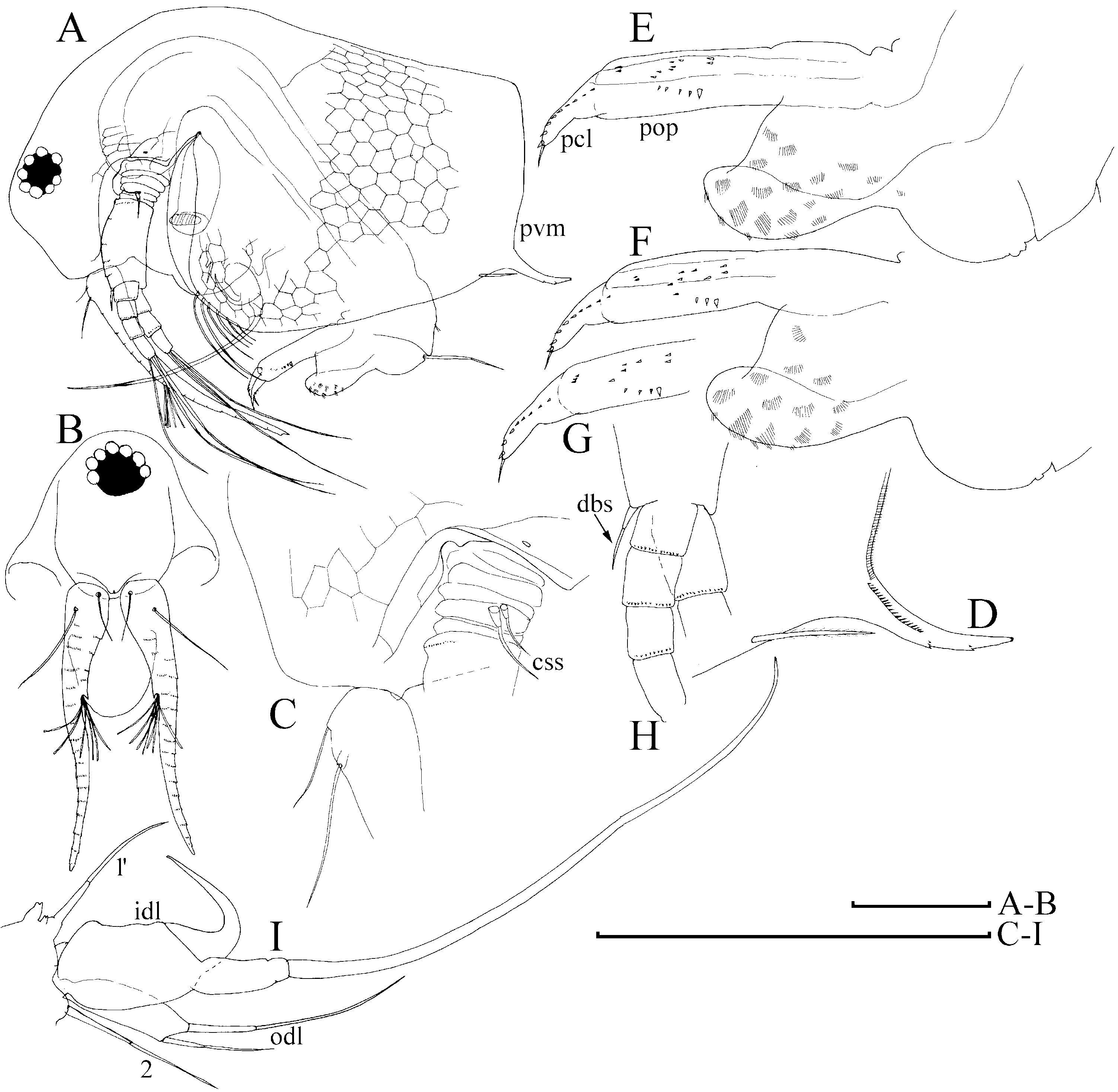
Diagnosis of adult male ( Figs 1 View Figure 1 , 2 View Figure 2 , 3A–D View Figure 3 ): Body elongated, humped, head elevated above dorsum of valve, dorsum posteriorly almost straight, posterior margin of valves (pvm) short, almost straight. Head large, anteroventral angle (ave) well-defined, but not projected as a rostral wrinkle; distalmost extremity of head slightly projected as an ocular dome (ocd). Frontal head pore (fhp) somewhat dorsally to base of antenna I, lateral head pore (lhp) immediately near, somewhat inflated lateral edge of head shield (as in female), median head pore (mhp) somewhat posterior to ocular dome. Mucro (muc) relatively long, with ventral incisions; seta kurzi (skz) long, a series on long setae (sav) at anteroventral portion of valve. Postabdomen elongated, its ventral margin (functionally oriented towards the top of the animal because abdomen strongly bent!) straight (vmp), preanal margin (prm) deeply depressed, distalmost portion of preanal margin projected distally, with numerous series of relatively long, fine, setules; anal margin (anm) straight, located in a depression. Postanal portion of postabdomen (pop) as long tube, blunt distally, supplied with groups of distinct denticles. Dorsalmost group (dmg) of few denticles apparently a remainder of proximal pecten of postabdomen in female (but, perhaps there are other denticles that are also homologous with this pecten). A solitary gonopore (gnp) opens distally. Postabdominal claw (pcl) shortened (as compared with female), but relatively slender for the subgenus, bent in middle, with pointed tip supplied with a distal spinule, distal pecten (dip) as a series of relatively robust denticles. Postabdominal setae (pos) shorter than preanal margin of postabdomen. Antenna I jointed with head, slightly S-shaped and regularly arched in anterior view, its base thick, pre-aesthetasc portion (prc) regularly narrowing distally, postaesthetasc (poc) portion thin, almost straight. Antennular sensory seta (ass) very long, located at a short distance from basal end of antenna I; male seta short (aml), located on a small pedestal near its base; nine aesthetascs (aes), slender, subequal in size. Anterior and lateral surface of antenna I with transverse series of small denticles. Antenna II as in female, but with two sensory setae on coxal part (css) (in contrast to female with a single seta), and one of them very long, reaching, or projecting behind, the middle of the basal segment. Distal anterior seta (dbs) of middle size (approximately as long as basal segment of three-segmented branch), located on anterior surface of distal segment, near distal end of distal segment. Limb I with additional groups of setules on posterior (i.e. inner) surface. Outer distal lobe (odl) as an elongated lobe with two setae of different size. Inner distal lobe (idl) strongly inflated, sometimes with a slight, smooth projection on basal portion, its distal portion bottle-shaped, terminating as a long, naked seta. Copulatory hook relatively small, not recurved to a parallel position with the idl, regularly tapering distally, tip of hook as a sharp spine, without setules. Subdistal lobe (sdl) slightly projected, with a single long, bisegmented seta (1′) and two closely located rudimentary setae. Seta 2 relatively long for genus, about half the length of more basal seta. Ejector hooks (ejh) of greatly different size.
Postembryonic development: Newborn males (and females) of Bosmina and other Anomopoda moult soon after (from several seconds to minutes) release from the brood pouch, and the first juvenile instar begins ( Kotov, 1996, 1997a; Kotov & Boikova, 2001).
Juvenile male I ( Fig. 4B–H View Figure 4 ) has a body shape similar to the juvenile female I. Posterior dorsal head pore (dhp) present only in females and males of instar I, fully disappearing in instar II. Postabdomen relatively similar to that in female ( Fig. 4A View Figure 4 ), but with preanal margin slightly depressed, ventral margin somewhat inflated, and paired rudimentary gonoduct visible through cuticle, although gonopore absent. Postabdominal claw as in female, with distal, proximal (pxp) and pre-claw (pcp) pectens on female type. Antennae I fused with rostrum, only a pair of sensory setae (which apparently are parts of antennae I) on ‘rostrum’, frontal head pore at level of these setae. Antenna II with two short sensory setae on coxal portion (in contrast to female with one seta), whereas a rudiment of distal anterior seta on basal segment absent. Limb I with odl of female type, idl (greatly reduced and lacking setae in female) small, subquadrangular, with a rudimentary seta, copulatory hook short and thick, subdistal lobe not projected, with a single seta 1 (as in female).
Juvenile male II ( Figs 3E–H View Figure 3 , 4I–P View Figure 4 ) has a body shape that is also similar to the juvenile female II, posterior dorsal head pore absent. Postabdomen with concave preanal margin and thick, tube-shaped postanal portion, and rudimentary gonoduct reaching half the length of postanal portion, although gonopore absent. Postabdominal claw shorter than in female II, distal pecten consists of denticles increasing in thickness proximally, proximal pecten shifted from claw lateral surface to postanal portion of postabdomen, consisting of numerous spinules, pre-claw pecten greatly reduced. Antennae I fused with rostrum, which is supplied with a pair of antennular sensory setae and a pair of male setae, somewhat dorsally to first pair, frontal head pore on middle line, dorsal to level of male setae. Antenna II with two sensory setae of unequal size on coxal portion, with a rudimentary distal anterior seta present. Limb I with odl of female type, idl large (but smaller than in adult), elongated, with a seta approximately as long as idl, and another rudimentary seta, copulatory hook longer, but thick, with triangular tip; subdistal lobe projected, with seta 1, and two other setae. Most probably, seta 1 is greatly reduced in size in adult males, whereas only seta 1′ is well developed.
As with chydorids, bosminids have two juvenile instars in male development. This instar number is fixed (apparently unaffected by food or other conditions). The transition to the adult (third) instar is usually accompanied by the greatest ontological changes in morphology. Just after this ecdysis, antenna I develops a joint separating it from the head, and all additional setae and hairs strongly increase in size ( Lilljeborg, 1901; Kotov, 1996). It is likely that the adult males lack a moult beyond instar III.
Comments: Males of B. longirostris have been described several times previously ( Sars, 1993; Alonso, 1996), but the postembryonic development remains unknown. Lilljeborg’s (1901) schematic illustrations of the male II habitus were not detailed enough to discuss the development of the male characters.
Uéno (1927) found a morphologically typical B. longirostris in Japan. But Tanaka (2000) described an adult male of ‘ B. longirostris ’ from Japan with a strange, extremely short and blunt postabdominal claw, and an exceptionally long copulatory hook on limb I. Populations from Japan must be re-examined; they may belong to another species (there is also a chance of introduction from North America, where the subgenus is more diverse). Chiang Sieh-chin & Du Nan-shan (1979) illustrated the male of B. fatalis or B. tripurae under the name ‘ B. longirostris ’.
De Melo & Hebert (1994) reported females of ‘ B. longirostris ’ with very fine and long setules on the base of the postabdominal claw from Hume Lake (California, USA). This determination seems to be dubious: perhaps this population belongs to a species that differs from B. longirostris sp. str. Indeed, our sampling from this location revealed specimens that group in the B. freyi clade. However, we have not compared the setules of specimens from the two sampling dates, and it is possible that we have sampled something different from the ‘ B. longirostris ’ described by De Melo & Hebert (1994).
Bosmina (Bosmina) liederi De Melo & Hebert, 1994 View in CoL , Figure 5 View Figure 5
Bosmina (Sinobosmina) liederi De Melo & Hebert, 1994: 1823 View in CoL ; fig. 13A–C.
Material: Artificially obtained males from the addition of MF in laboratory culture, initially from Bass Lake, CA, USA, AAK 2004-092.
Differences from B. (B.) longirostris: Body less humped, posterior margin of valve high. Denticles in all series on postanal portion of postabdomen: minute. Postabdominal claw thick, with relatively blunt tip [supplied with a distal spinule, as in B. (B.) longirostris ]. Antenna I characteristically S-shaped in anterior view. Antenna II with short sensory setae on coxal portion, and a short distal anterior seta (not reaching distal end of the first endopod segment, or distal end of the second exopod segment).
Comments: Unfortunately, only a single population of B. (B.) liederi , well-differentiated from B. (B.) longirostris genetically, and with artificially induced males, was studied. Although Kim et al. (2006) found that artificial induction leads to the appearance of morphologicaly normal, instead of monstrous, males, our list of differences between species must be checked by examination of several other populations of B. (B.) liederi .
De Melo & Hebert (1994) erroneously placed this species in the subgenus Sinobosmina , based on information on the mobility of the allozyme products of a few alleles in North American species from different subgenera. Subsequently, Taylor et al. (2002) proposed that B. liederi is a member of the subgenus Bosmina s. str., and the sister species to B. (B.) longirostris . Our expanded sampling of this clade (using specimens from the type region and from Asia) and detailed morphological analyses support the sister-species relationship of B. (B.) liederi and B. (B.) longirostris .
Bosmina (Bosmina) tripurae Kořínek, Saha & Bhattacharya, 1999 View in CoL
Bosmina cf. japonica Poppe & Richard, 1890 View in CoL in Kořínek, 1971: 292–294; figs 10F–H, 11A–D.
Bosmina tripurae Kořínek et al., 1999: 241–247 View in CoL ; figs 1A–D, 2A–F, 3A–G, 4A–D.
Not Bosmina japonica Poppe & Richard, 1890: 76–77 View in CoL .
Source of information: The description of Kořínek et al. (1999). Some important details (i.e. gonopore position and pattern of denticles on postanal portion of postabdomen) are not described or illustrated.
Diagnosis of adult male: Body relatively high, not humped, dorsum in posterior half slightly convex, posterior margin especially high. Head large, anteroventral angle well defined, but not projected; distalmost extremity of head slightly projected as an ocular dome. Frontal head pore somewhat dorsal to base of antenna I, lateral head pore at a small distance from lateral edge of head shield (as in female). Mucro relatively long, seta kurzi long, a series of long setae at the anteroventral portion of valve. Postabdomen elongated, its ventral margin slightly convex, preanal margin moderately depressed, distalmost portion of preanal margin (dorsodistal angle) not projected distally, with numerous series of relatively long, fine, setules; anal margin straight, not within a depression. Postanal portion massive, blunt distally, supplied with groups of denticles of pattern unclear from the author’s illustrations. Dorsalmost group of about seven or eight denticles, a remainder of a proximal pecten of the postabdominal claw, apparently continues as a distal pecten on postabdominal claw. Gonopore not illustrated. Postabdominal claw relatively thin, slender, regularly curved, with distal spinule, and with distal pecten as a series of robust denticles. Antenna I articulated at the attachment site to the head, probably straight in anterior view, its pre-aesthetasc portion regularly narrowing distally, post-aesthetasc portion thin, almost straight. Antennular sensory seta short, located at a distance from base; male seta short, located on a minute pedestal. Antenna II with two sensory setae on coxal part, which are shorter than sensory setae of B. (B.) longirostris . Distal anterior seta short, as long as basal segment of four-segmented branch. Limb I with additional groups of setules on inner surface. Strongly inflated idl, with a slight projection on basal portion, its distal portion bottle-shaped, terminating as a long, naked seta. Copulatory hook relatively small, recurved to a parallel position with the idl, regularly tapering distally, tip of hook blunt, with a ridge. Subdistal lobe large, with single long seta and two closely located rudimentary setae. Ejector hooks different in size by 1.5 times.
Comments: The species was first described as B. cf. japonica Poppe & Richard, 1890 from India ( Kořínek, 1971), but Kořínek stated that the male of this animal is markedly different from males of B. (B.) longirostris and B. (S.) fatalis . Kořínek et al. (1999) described the adult male of this species, and placed it in the subgenus Sinobosmina , based on the position of the lateral head pore, structure of male postabdomen, and copulatory hook. But, according to our re-evaluation of the genus system, we reveal that this species is a primitive member of the subgenus Bosmina sp. str., instead of Sinobosmina . Most of the differentiation of B. (B.) tripurae from B. (B.) longirostris , i.e. unhumped body, high posterior margin, massive postanal portion of postabdomen, absence of an anal depression, proximal pecten apparently continues as distal pecten on postabdominal claw, short sensory setae on coxal part, and short distal sensory seta on basal segment of antenna II, and smaller difference in size of ejector hooks, are apparent plesiomorphies of B. (B.) tripurae .
| V |
Royal British Columbia Museum - Herbarium |
| CA |
Chicago Academy of Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Bosmina (Bosmina)
| Kotov, Alexey A., Ishida, Seiji & Taylor, Derek J. 2009 |
Bosmina tripurae Kořínek et al., 1999: 241–247
| Korinek V & Saha RK & Bhattacharya T 1999: 247 |
Bosmina (Sinobosmina) in De Melo & Hebert, 1994: 1820
| De Melo R & Hebert PDN 1994: 1820 |
Bosmina (Sinobosmina) liederi
| De Melo R & Hebert PDN 1994: 1823 |
Bosmina (Bosmina) longirostris (O. F. Müller)
| Lieder U 1983: 126 |
| Negrea S 1983: 219 |
| Flossner D 1972: 214 |
| Keilhack L 1909: 52 |
Bosmina cf. japonica
| Korinek V 1971: 292 |
Bosmina (Bosmina)
| Taylor DJ & Ishikane CR & Haney RA 2002: 1494 |
| De Melo R & Hebert PDN 1994: 1818 |
| Lieder U 1983: 123 |
| Lieder U 1962: 317 |
Garbinia
| Grochowski M 1910: 343 |
Garbinia adriani
| Grochowski M 1910: 343 |
Bosmina pellucida
| De Melo R & Hebert PDN 1994: 1818 |
| Stingelin T 1895: 229 |
Bosmina japonica
| Poppe SA & Richard J 1890: 77 |
Bosmina longirostris (O. F. Müller)
| Sars GO 1993: 79 |
| Margaritora FG 1985: 56 |
| Margaritora FG 1983: 32 |
| Sramek-Husek R & Strascraba M & Brtek J 1962: 277 |
| Burckhardt G 1941: 130 |
| Ueno M 1927: 285 |
| Keilhack L 1908: 444 |
| Lilljeborg W 1901: 225 |
| Muller PE 1867: 146 |
| Baird W 1850: 105 |
Bosmina
| Baird W 1846: 412 |
| Baird W 1845: 149 |
Eunica
| Koch CL 1841: 23 |
Lynceus longirostris O. F. Müller, 1776: 199
| Muller OF 1785: 76 |
| Muller OF 1776: 199 |