Cryptosporidium viatorum, Elwin et al., 2012
|
publication ID |
https://doi.org/10.1016/j.ijppaw.2018.01.004 |
|
DOI |
https://doi.org/10.5281/zenodo.10892170 |
|
persistent identifier |
https://treatment.plazi.org/id/03C64155-FF9A-5465-FCA4-2CDA77C64088 |
|
treatment provided by |
Felipe |
|
scientific name |
Cryptosporidium viatorum |
| status |
|
3.3. Molecular identification and classification as C. viatorum
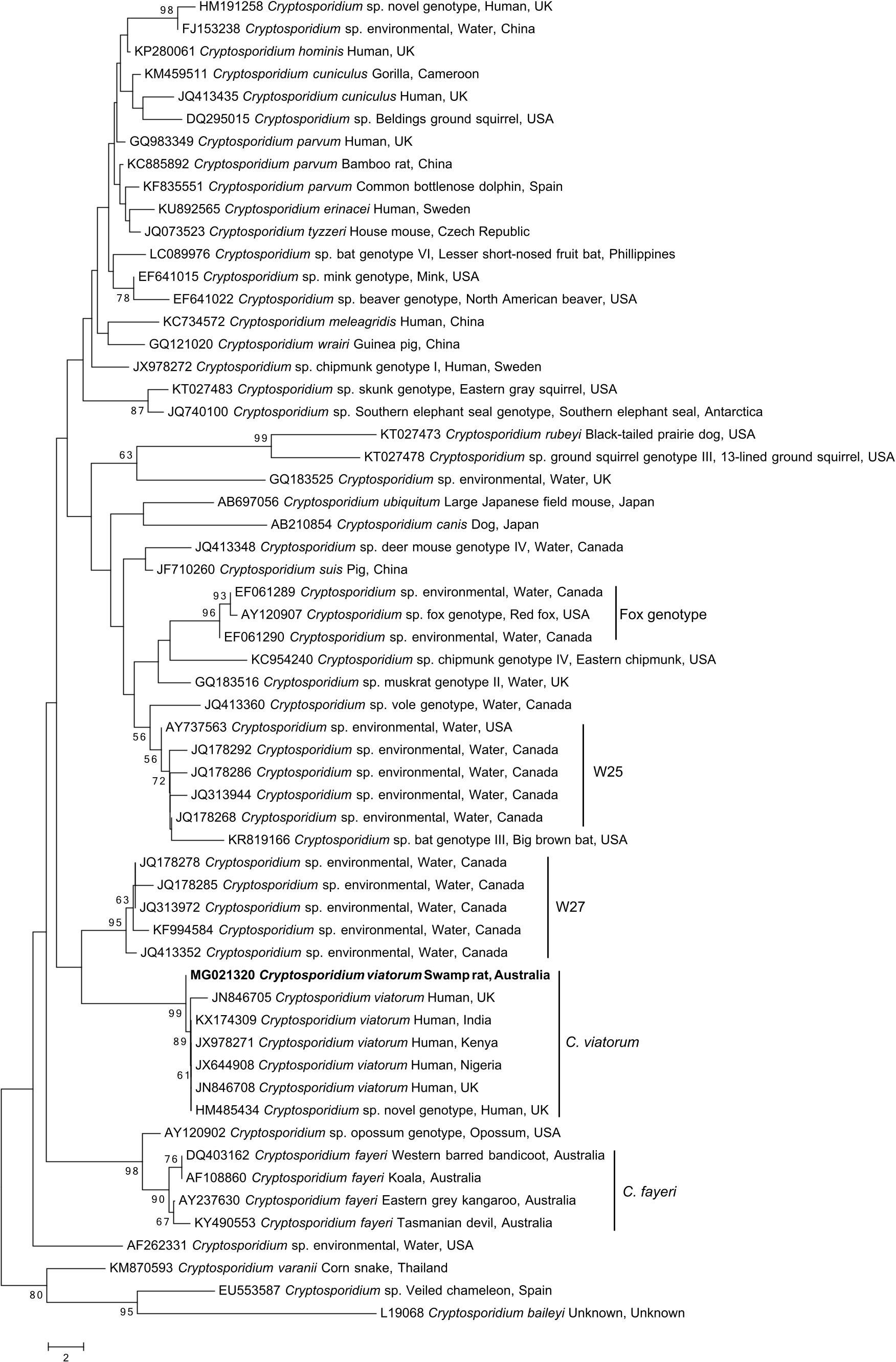
Of the 26 rat faecal samples examined, three from R. lutreolus (laboratory nos. UY 7513, UY7521 and UY 7523) (but none from R. fuscipes or M. fuscus ) were PCR test-positive for Cryptosporidium by SSU. The SSU sequences determined (represented by accession no. MG 021320) were all identical across all 568 nts; a comparison of this novel sequence with the sequence representing C. viatorum (accession no. JN846708) from GenBank revealed 99% identity (four deletion/insertion events). Only one of the gp60 amplicons derived from rat faecal DNAs ( UY 7513) returned a clean gp60 sequence for Cryptosporidium of 911 bp in length. This sequence (accession no. MG 021319) had 84% identity (767 of 911 bp) with C. viatorum (accession no. KP 115936) present in GenBank at the time.
The SSU phylogenetic tree ( Fig. 1 View Fig ) suggests that the Cryptosporidium taxon in the faecal sample from R. lutreolus is most closely related (537 of 541 bp or 99.3% identity) to the majority of C. viatorum sequences. The sequences of C. viatorum grouped into a highly-supported monophyletic clade (BP = 99%). Five of the previously defined C. viatorum sequences (accession nos. KX 174309, JX978271, JX644908, JN846708 and HM 485434) are identical, while one (JN846705) has a single nucleotide difference to the other five sequences. The clades that are closest to the C. viatorum clade in the tree are a well-supported clade (BP = 95%) containing multiple sequences representing Cryptosporidium sp. environmental water samples from Canada ( Ruecker et al., 2012; 2013; Prystajecky et al., 2014) and the strongly-supported (BP = 98%) marsupial clade representing sequences of C. fayeri and Cryptosporidium sp. opossum genotype ( Morgan et al., 1999; Xiao et al., 2002; Power et al., 2004; Wait et al., 2017).
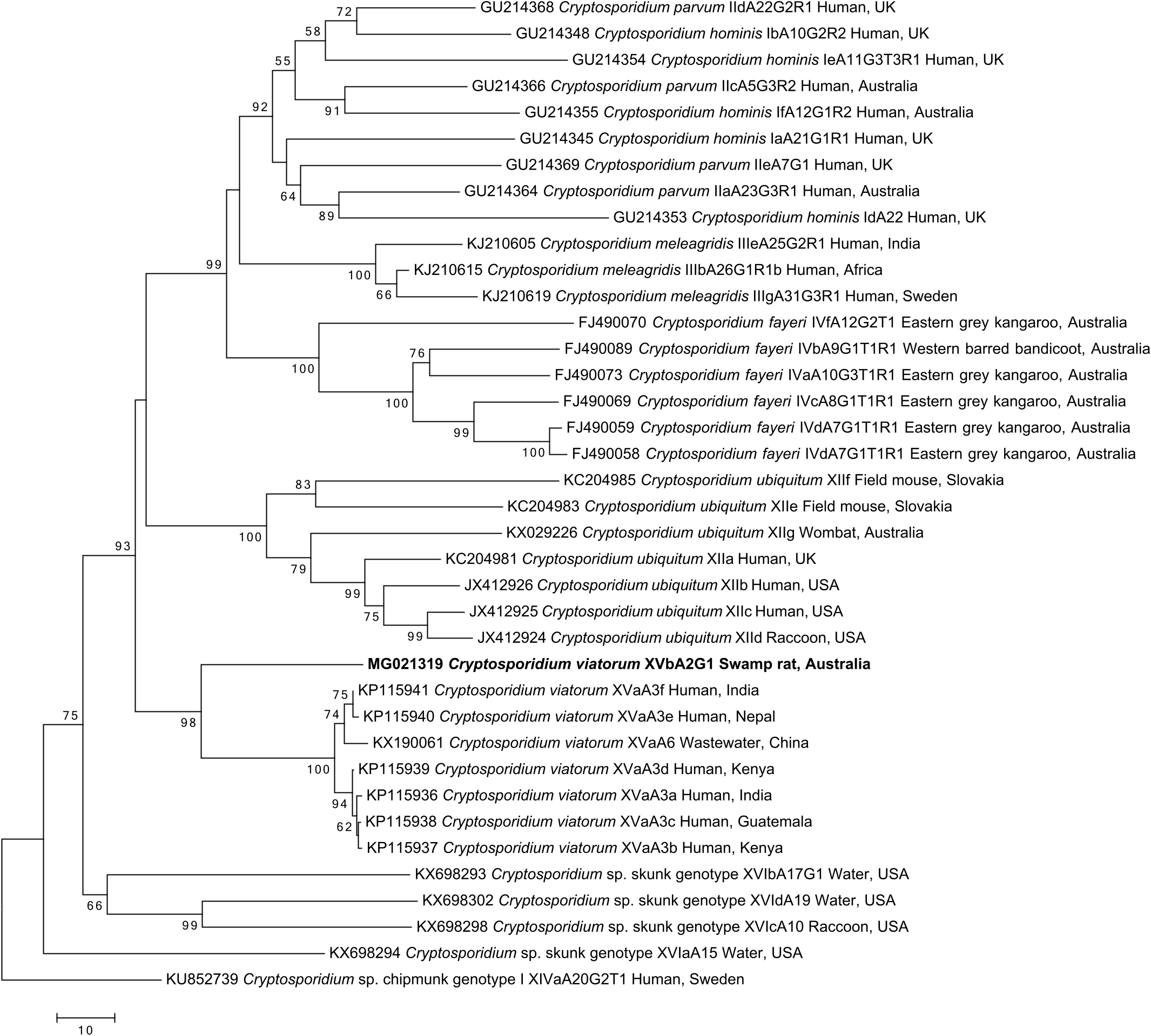
The gp60 tree ( Fig. 2 View Fig ) also reveals a strongly supported monophyletic C. viatorum clade (BP = 98%). Six members of the previously recognised subtypes XVaA3a to XVaA3f ( Stensvold et al., 2015) as well as XVaA6 from wastewater ( Huang et al., 2017) appear to cluster tightly in a well-supported clade (BP = 100%) with relatively short branch lengths. The difference in branch length between the novel C. viatorum sequence and the other C. viatorum sequences (accession nos. KP 115936 to KP 115941) leads to the designation of a new subtype XVb.
All of the other Cryptosporidium species and genotypes group into well supported clades, with the exception of C. parvum and C. hominis which remain together within their own clade. Of note is the extent of the maximum level of intraspecific variation recorded to date within C. meleagridis (16.3%), C. fayeri (37.8%), C. ubiquitum (54.3%) and Cryptosporidium sp. skunk genotype (45.6%), compared to that within C. viatorum (26.3%).
3.4. Comparison of gp60 and sequence data
The nucleotide alignment of gp60 (not shown) indicates that the descriptive serine repeat region contains two TCA serine repeats followed by a third TCG serine repeat which, in accordance C. viatorum subtype naming guidelines ( Stensvold et al., 2015), designates the name of the novel C. viatorum subtype as XVbA2G1. The pairwise nucleotide sequence difference of the novel subtype to the other C. viatorum subtypes ranged from 16.3% to 17.3% (Table 1). The intrasubtype variation for the known subtypes ranges from 0.1 to 3.7% difference.
Pairwise comparisons showed 8 amino acid deletions, 6 insertions and 53 non-synonymous substitutions when the C. viatorum subtype XVbA2G1 was compared with other C. viatorum subtypes (Fig. 3), with variation ranging from 22.0% to 24.8% across the consensus length of 286 amino acids. Variation among six other known subtypes ranged from 0.4% to 6.0% at the amino acid level.
The “diagnostic” serine repeat region (Fig. 3: amino acid postions 26–32) precedes a threonine repeat region, which includes 10 repeats of threonine with an alanine in the middle of the C. viatorum subtypes from humans but is lacking from subtype XVbA2G1 from the swamp rat (Fig. 3). The furin cleavage site (“RAKR”) in the sequence of subtype XVbA2G1 was at the same position as in C. viatorum subtypes from humans or from wastewater (“IVKR”), but there were two amino acid differences (Fig. 3). In addition, subtype XVbA2G1 differs at two of the recognised N-glycosylation sites from the other subtypes: the first Nglycosylation site is 7 amino acids further downstream, and the second site is at the same position but its sequence is “NETD” rather than “NDSD” or “NDND”. Of the 35 O-glycosylation sites predicted for subtype XVbA2G1 and all other known C. viatorum subtypes, 23 amino acid positions (66%) were conserved among subtypes.
4. Discussion
Since the initial description of C. viatorum from travellers returning to the United Kingdom from the Indian subcontinent, there has been a question as to whether animal reservoirs exist for C. viatorum ( Elwin et al., 2012) . This question has at least partially been resolved with the discovery of C. viatorum subtype XVbA2G1 from native Australian swamp rats living in an isolated and protected water catchment free from human intervention and in which other wildlife have been continuously surveyed for Cryptosporidium since 2009 ( Nolan et al., 2013; Koehler et al., 2016b). Although this subtype is not identical to those found in humans ( Stensvold et al., 2015), the molecular and phylogenetic evidence provided here indicates they it belong to the same clade as other subtypes and thus likely represents the same species of Cryptosporidium .
In the following, we (i) provide support for the molecular identification of C. viatorum from R. lutreolus and discuss its relationship to currently known C. viatorum subtypes from humans; (ii) give an historical account of rats in Australia and their role or potential role as hosts for Cryptosporidium ; and (iii) discuss the epidemiological context for C. viatorum in Australia and its zoonotic potential.
4.1. Molecular identification of C. viatorum
The sequence differences between the novel subtype (XVbA2G1) and the human C. viatorum subtypes for the SSU gene is only four nucleotides (out of 541) or 0.7% difference ( Fig. 1 View Fig ), which borders on the acceptable limits of intraspecific variation for Cryptosporidium ( Ruecker et al., 2012; Ryan et al., 2014; Koehler et al., 2016a). Convincingly, subtype XVbA2G1clearly clusters together in a well-supported monophyletic clade with the other C. viatorum sequences from humans ( Fig. 1 View Fig ). The inclusion of the majority of unique Cryptosporidium sequences, from the top half of the Cryptosporidium phylogeny (cf. Ruecker et al., 2012), provides further evidence that there is no sequence currently publicly available on GenBank to which the novel subtype matches closer than to the known C. viatorum subtypes from humans ( Fig. 1 View Fig ). The closest clades to the C. viatorum clade are the Canadian environmental sequences (collectively referred to as W27 ( Ruecker et al., 2012)), taken from water samples, and the marsupial clade, which includes the Australian C. fayeri and the North American opossum genotype ( Fig. 1 View Fig ). Unfortunately, the host(s) from which the Canadian environmental water samples originated is unknown ( Ruecker et al., 2012). The common locality of Australia (between the marsupial clade and the novel subtype) is interesting, but there is currently not enough information available to lend significant support towards any conjectures about the evolutionary relationship between C. viatorum and the other clades.
The gp60 gene provides clear evidence that XVbA2G1 is a subtype of C. viatorum . The gp60 tree ( Fig. 2 View Fig ) suggests a strongly supported monophyletic clade for C. viatorum when compared to the other Cryptosporidium species for which there are gp60 sequences in GenBank ( Strong et al., 2000; Power et al., 2009; Li et al., 2014; Stensvold et al., 2014, 2015; Huang et al., 2017; Yan et al., 2017). The intraspecific variability of all the other major clades of Cryptosporidium species included within the gp60 tree ( Fig. 2 View Fig ) is comparable to that seen within the C. viatorum clade. Indeed, sequence variation among representatives within species (clades) are usually higher than for C. viatorum , except for C. meleagridis ( Fig. 2 View Fig ).
The tight grouping of C. viatorum subtypes XVaA3a - XVaA3f could be suggestive of a recent spread of C. viatorum from a source population, which is now being disseminated via global human travel, as there does not appear to be a geographic partitioning according to country ( Fig. 2 View Fig ). A more extensive study would be necessary to support this hypothesis, although a population study of gp60 from C. parvum in humans reported that the reduced level of variation within C. parvum could represent a recent adaptation to the human host or a selective sweep ( Abal-Fabeiro et al., 2013).
As mentioned previously, the subtype XVbA2G1 is not identical to other C. viatorum subtypes from humans or wastewater, but it is considered similar enough to represent C. viatorum . The genetic difference at the gp60 locus between subtypes is significant enough to suggest geographic isolation between C. viatorum from Australia and C. viatorum from other locations as a possible explanation. Isolation of the Australian subtype coupled with a relatively recent global spread of the human subtypes could also account for these differences; however, many more samples would need to be tested before a definitive conclusion could be made regarding population structure for C. viatorum .
The alignment of the inferred GP 60 amino acid sequence data of C. viatorum subtypes further supports the conjecture that subtype XVbA2G1 belongs to C. viatorum based on the conserved the furin cleavage site and the partial conservation of the N and O-glycosylation sites (Fig. 3) (cf. Stensvold et al., 2015). Additionally, the unique serine repeat region, which has been the cornerstone of Cryptosporidium genotyping ( Strong et al., 2000; Sulaiman et al., 2005), is followed by a threonine repeat region (Fig. 3). Thus far, C. viatorum is the only species of Cryptosporidium that has a threonine repeat region rather than a truncated serine repeat ( Stensvold et al., 2015). In the future, this region of threonine repeats might be useful to assess the classification of novel subtypes of C. viatorum , in addition to the considerably shorter serine repeat region.
| SSU |
Saratov State University |
| MG |
Museum of Zoology |
| HM |
Hastings Museum |
| GP |
Instituto de Geociencias, Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |