Myocastor coypus (Molina, 1782)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6623649 |
|
DOI |
https://doi.org/10.5281/zenodo.6620171 |
|
persistent identifier |
https://treatment.plazi.org/id/03C5A071-FFE3-FFD6-FAC5-55005D02F6AC |
|
treatment provided by |
Carolina |
|
scientific name |
Myocastor coypus |
| status |
|
Coypu
French: Ragondin / German: Nutria / Spanish: Coipu
Other common names: Nutria
Taxonomy. Mus coypus Molina, 1782 ,
“Chili.” Restricted by C. A. Woods and colleagues in 1992 to “Rio Maipo, Santiago Province, Chile.”
Myocastor coypus includes bonariensis, castoroides, popelairi, chilensis, albomaculatus, dorsalis, santaecruzae, and melanops as synonyms. Four subspecies recognized.
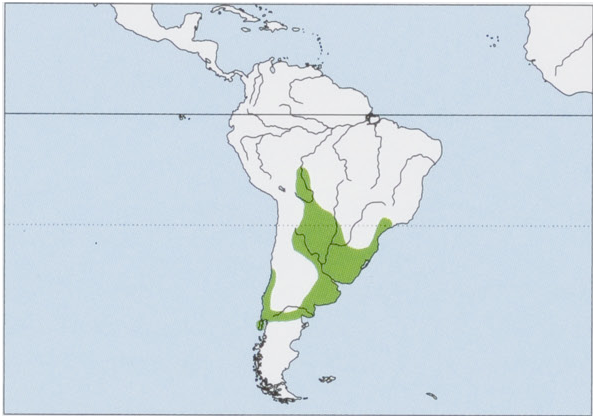
Subspecies and Distribution.
M.c.coypusMolina,1782—Chilemainland.
M.c.bonariensisE.GeoffroySaint-Hilaire,1805—Bolivia,Paraguay,NArgentina,SEBrazil,andUruguay.
M.c.melanopsOsgood,1943—ChiloéI(CChile).
M. c. santacruzae Hollister, 1914 — Argentina , from the Chaco S to Patagonia. Introduced widely into N South America, S North America, Europe, Central Asia, and East Africa. View Figure
Descriptive notes. Head-body 472-575 mm,tail 340-405 mm; weight up to 6-7 kg. The Coypu is large-bodied, robust, heavy, and adapted for an aquatic lifestyle. Ears are small, nearly hidden by thick fur; mouth is closable behind incisors;tail is ¢.72% of head-body length, round,thick, scaly, and sparsely haired; limbs are short; forefeet with five clawed digits, but pollex greatly reduced; hindfeet with well-developed claws on all five digits, webbing joining first four digits, fifth digit unwebbed and used for grooming; and pelage is soft and thick, with dense underfur covered by elongated guard hairs. Sebaceous glands are located near corner of mouth and near anus. Female Coypus have four pair of mammae, each lateral in position and located high on sides. Skull is long and broad, flat in lateral profile, and has broad, deep, and elongated rostrum. Sagittal crestis well developed; zygomatic arches are broad and flaring; jugal is thick but does not approach lacrimal; andjugal fossa is reduced. Infraorbital foramen is large, without distinct groove on ventral surface, indicative of passage of infraorbital branch of maxillary nerve. Stems of internal carotid artery and stapedial artery are missing. Angular process of mandible is strongly deflected, and coronoid processis vestigial. All cheekteeth, above and below, are flatcrowned and extremely hypsodont but not hypselodont. Upper tooth rows converge anteriorly but not to the degree as in species of Dactylomys , and they are inclined outward. Upper cheekteeth have two labial and three lingual folds; lower cheekteeth have one labial and three lingual folds, although dP, has an additional anterior lake (fossette). Re-entrantfolds of upper and lower cheekteeth become lakes with wear. Incisors are broad and deep, with enamel strongly pigmented orange. Chromosomal complement is 2n = 42 and FN = 76.
Habitat. Slow-moving streams, lakes, swamps, freshwater marshes,irrigation channels, and even brackish water, with sufficient succulent vegetation. Coypus are aquatic; they are semi-fossorial and build complex burrows. They are primarily a lowland animal but may occur up to 1200 m in the Andes.
Food and Feeding. Coypus feed preferentially on semi-aquatic and aquatic plants but opportunistically may consume waterside terrestrial vegetation, especially in winter. Dietary items include stems, leaves, roots, and sometime bark; they sometimes use immersed branches or logs as feeding platforms. Forepaws are used to manipulate and hold food items. In Chile, Coypus consumed 700-1500 g (mean 1100 g) of plant matter per day, which represent daily intake equalto ¢.25% of body mass. Where populations occur near agricultural plots, individuals can be responsible for considerable damage by consuming cereals or other cultivated plants. Coypus are coprophagous in natural and captive situations, an activity usually performed at night.
Breeding. Given large body mass and shortlife span (averaging 6-3 years), the Coypu has long gestation of 127-139 days. Age atfirst parturition is 6-15 months; parturition is usually followed by estrus within two days postpartum, with mean postpartum interval of 2-1 weeks. The Coypuis polyestrous, and length of estrus is 5-60 days, with some healthy females showing no sign of estrus over the course of several months. This variation may be related to ovulation induced by coitus. Cholesterol and ketosteroid levels increase incrementally as ovaries of young females develop. During estrus, amounts of these hormones increase in adrenal cortex and ovary. In pregnant females, these hormones are present only in very small amounts. There are 3-17 corpora lutea, usually a higher number than actual implanted embryos, suggesting that the Coypu is polyovular. In introduced populations in the USA, Coypus are non-seasonal breeders, with peak birth rates in December—January and June-July in Louisiana and January, March, May, and October in Oregon. Mean litters in Oregon had 3-6 young (maximum 1-12 young), with a decline in winter. Litters are larger in regions with higher habitat productivity. For unknown reasons, embryos are almost always implanted in left horn of the uterus. Mean body mass of young at birth is nearly 225 g, and neonates rapidly grow during the first five months oflife (growth rates of 0-0116-0-0120 g/day). Neonatal growth rate may be affected by temperature and habitat disturbances. Lactation of feral Coypu in England lasts 7-7 weeks. In captivity, young nursed for c¢.2 months and were able to survive if weaned after 32 days. Milk of the Coypu contains 41:5% dry matter, 27-9% fat, 13-7% protein, and 0-5% sugar, with the remainder being ash. In Maryland, USA, annual productivity was 8-1 young/female,likely related to food availability and abundance, predator abundance, weather conditions, and diseases. When environmental conditions remain stable and good, meanlittersize was 2-7 young, and females produced an average of 15 young/year. Females Coypus may “practice” birth control by spontaneous abortion when litter size is small or iflitter consists only of female embryos. In these cases, link between embryo disc and decidua basalis is destroyed, leading to death of an embryo and its disc destruction (up to 25% reabsorptions).
Activity patterns. The Coypu is essentially nocturnal, with activity usually initiated at dusk and ending before daylight in early morning. Most daily activity is spent feeding, swimming, and grooming. During hottest period of the day, Coypus sleep in their burrows, in the sun, in the water, or on their feeding platforms. Individuals build burrows with multiple entrances; some tunnels are 6 m in length (range 1-6 m).
Movements, Home range and Social organization. Individual Coypus usually stay in only one area throughout their lives, but some local emigration has been observed during freezing weather in particularly harsh winters. Otherwise, daily movements are typically less than 45 m; 50% of tagged males were recaptured within a radius of 91-4 m; 80% of all movements were within a radius lesser than 400 m and 20% within radii of 400-1250 m. Radio-collared individuals during a two-year study in the Netherlands moved no more than 300 m in water and 50 m on land, but more extreme movements were observed in young adults, who moved as far as 120 m downstream. Densities were 0-1 ind/ha in unfavorable habitats and 25 ind/ha in more swampy or riverine habitats. A study in France revealed that 40% of suitable habitats was unoccupied in winter. Population size was largely affected by winter cold, affecting reproduction success and adult survival. In suitable French habitats, local densities were 9-1 ind/ha in May and 2-4 ind/ha in November. Densities have also been correlated with habitat quality, especially local population levels, in Florida, where densities were 24-7 ind/ ha in unpolluted waterways but only 5-9 ind/ha in sewage polluted ponds. Within their native distribution in north-eastern Argentina, Coypuslive in social groups with a mean of eleven individuals per group; in captivity, group sizes are 3-13 individuals. These groups usually have one dominant adult male, with several socially subordinated adults, subadult females and males, and juveniles. Most subordinated individuals are related offspring from a single female. Some subgroups of subadults and juveniles were also recorded. Alpha males and alpha females have been identified in several studies, with the female apparently dominant over the male except when pregnant or lactating young. Young adults are frequently expelled from groups and might be occasionally found as solitary individuals. Several amicable and cooperative behaviors are commonly observed among Coypus . They are believed to have polygynous mating system, with a dominant male mating with multiple females per social group. In France, female home range was determined to be quite constant (2-5-5-9 ha), despite density variation, and with broad individual overlap. A similar pattern was observed in Louisiana where home ranges were 2:5-5-7 ha.
Status and Conservation. Classified as Least Concern on The [UCN Red List. The Coypu is widespread and abundant within its native habitat in southern South America. At high density, Coypus can damage agricultural crops but only limitedly. It is also regarded as a pest in some regions due to destruction ofriverbanks, disruption of drainage systems, damage to crops (e.g. rice, sunflower, corn, cowbane, great water dock, and diverse crop roots), and damage to native plant communities. Its wide distribution in its native and introduced lands, occurrence in several national parks over in its native distribution but also in Europe and North America support the current listing. Due to its invasive nature, several eradication programs have been implemented in Palearctic and Nearctic areas but not in its native distribution. Invasive status of the Coypu has made it one of the most studied species of echimyids.
Bibliography. Abbas (1991), Bertolino et al. (2005), Borgnia et al. (2000), Bounds (2000), Brown (1975), Burrow (1815), Carter & Leonard (2002), Colares et al. (2010), Corriale et al. (2006), DAdamo et al. (2000), Desmarest (1826), Doncaster & Micol (1989, 1990), Ehrlich (1966, 1967), Felipe et al. (1998), Fischer (1829), Fitzinger (1867), Geoffroy Saint-Hilaire (1803, 1804, 1827), Gosling (1974, 1979, 1981), Gosling & Baker (1982), Gosling, Baker & Clarke (1988), Gosling, Guyon & Wright (1980), Gray (1827), Guichén & Cassini (2005) , Guichén, Benitez et al. (2003), Guichén, Borgnia et al. (2003), Harris & Webert (1962), Hollister (1914a), llliger (1815), Kerr (1792), Lesson (1842), Link (1795), Lund (1840, 1841), Lund & Couto (1950), Marelli (1931), Molina (1782) , Newson (1966), Oken (1816), Osgood (1943), Reggiani, Boitani, D’Antoni & De Stefano (1993), Reggiani, Boitani & De Stefano (1995), Thomas (1881), Wesmael (1841), Willner et al. (1979), Woods & Howland (1979), Woods & Kilpatrick (2005), Woods et al. (1992).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.