Brumoides suturalis (Fabricius), 1798
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5378.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:68976F75-EC46-480B-AB8A-061B1441A958 |
|
DOI |
https://doi.org/10.5281/zenodo.11067902 |
|
persistent identifier |
https://treatment.plazi.org/id/03C44153-FFB4-FFB4-FF77-FCE8FC5AF821 |
|
treatment provided by |
Plazi |
|
scientific name |
Brumoides suturalis (Fabricius) |
| status |
|
Brumoides suturalis (Fabricius)
( Figs 17–21 View FIGURE 17 View FIGURE 18 View FIGURE 19 View FIGURE 20 View FIGURE 21 )
Coccinella suturalis Fabricius, 1798: 78 .
Brumus suturalis : Mulsant 1850: 494; Korschefsky 1932: 267.
Brumoides suturalis : Chapin 1965: 237; Poorani 2002: 310.
Brumus daldorfii Crotch, 1874: 21 .
Brumoides daldorfii : Kovář 2007: 592; Dorji et al. 2019: 503.
Brumoides kolhapurensis Sathe & Bhosale, 2001: 28 . Synonymized by Poorani 2005: 186.
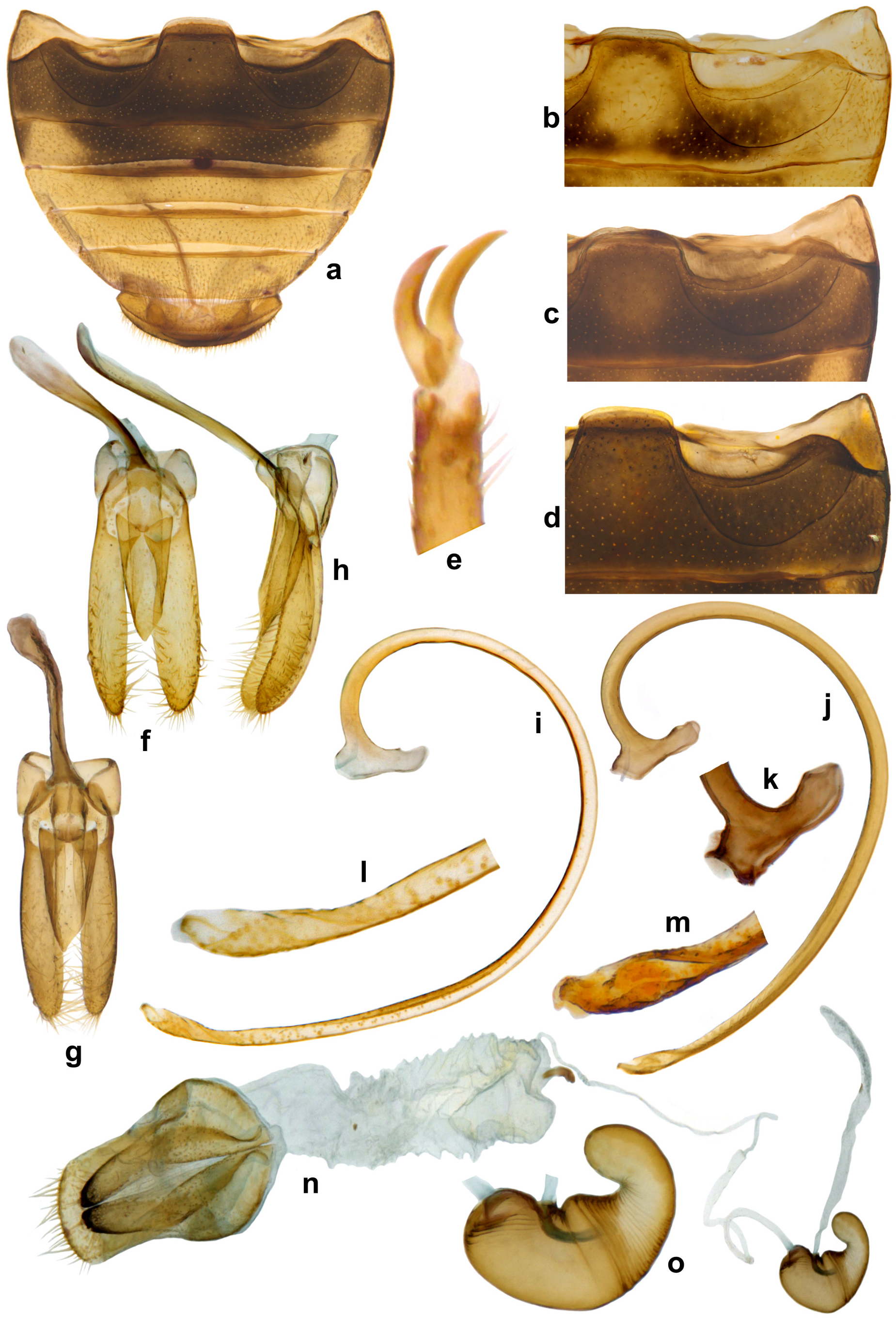
Diagnosis. Length: 3.50–3.80 mm; width: 2.70–3.00 mm. Form ( Fig. 17a–d View FIGURE 17 ) oval, dorsum convex. Head and pronotum orange yellow. Scutellar shield black. Elytra satiny white to creamy yellow, with three black vittae / stripes, one on each elytron in a mid-dorsal position not extending to apex and one sutural stripe not extending to apex, apical portion yellowish to reddish brown. Ventral side yellow except metaventrite and first abdominal ventrite dark brown to black, ventrite 2 dark brown except lateral sides paler, yellowish. Legs fully yellow or variable as follows: yellow except 3rd and 4th tarsomeres of all legs brownish, basally and apically darker brown to black, hind coxae more or less black, hind femora medially black, metatibiae and occasionally mesotibiae with outer margins black. Maxilla with terminal palpomere yellow except posterolateral corners dark brown to black. Anterior clypeal margin medially emarginate. Antenna ( Fig. 17f View FIGURE 17 ) 8-segmented, short, antennomeres distinctly wider than in B. lineatus , terminal antennomere often dark brown apically. Interspaces between punctures on head and pronotum with reticulate sculpture, those on elytra with microsculpture. Tarsal claws simple ( Fig. 18e View FIGURE 18 ). Abdominal postcoxal line complete, of somewhat variable depth ( Fig. 18a–d View FIGURE 18 ). Last visible abdominal ventrite posteriorly emarginate in male and narrowly rounded in female. Male genitalia ( Fig. 18f–m View FIGURE 18 ) as illustrated, penis guide distinctly shorter and reaching only a short distance beyond middle, apically asymmetrical ( Fig.18f, g View FIGURE 18 ); penis with a prominent capsule ( Fig. 18i, j, k View FIGURE 18 ), penis apex ( Fig. 18l, m View FIGURE 18 ) as illustrated. Female genitalia ( Fig. 18n View FIGURE 18 ) with a characteristic infundibulum distinctly longer and tubular; spermatheca ( Fig. 18o View FIGURE 18 ) as illustrated.
Externally similar to B. lineatus and can be differentiated from it by the slightly more elongate body outline, distinctly narrower elytral vittae (less than half as wide as elytral width), antennae with broader antennomeres and the genitalia of both sexes are also diagnostic.
Life stages. Life stages as illustrated ( Figs 19 View FIGURE 19 , 20 View FIGURE 20 ). Eggs ( Fig. 19a View FIGURE 19 ) creamy white to pale yellow with distinct microsculpture on chorion. Larva ( Fig. 19b–d View FIGURE 19 ) pale yellowish to darker yellowish brown or grey ( Fig. 20a–f View FIGURE 20 ), with prominent, blackish thoracic plates and spiny dorsal protuberances. Pupa ( Figs 19e View FIGURE 19 , 20g, h View FIGURE 20 ) variable in coloration, pale yellow to much darker.
Distribution. Widespread almost throughout India (Andhra Pradesh; Goa; Jammu & Kashmir; Karnataka; Kerala; Manipur; Punjab; Tamil Nadu; Uttar Pradesh; Uttarkhand; West Bengal). Nepal. Bhutan. Sri Lanka. Pakistan. Introduced and established in parts of Hawaii.
Prey/associated habitat. Polyphagous and feeds on aphids, whiteflies, psyllids, scales, mealybugs and mites. Gorham (1894) reported its feeding on pollen of grasses.
Specific host records are as follows: Hemiptera : Aleyrodidae : Aleurocanthus woglumi Ashby , Aleurolobus barodensis (Maskell) , Aleurolobus citrifolii Corbett , Bemisia tabaci (Gennadius) , Dialeurodes elongata Dozier , Dialeurodes citri (Ashmead) , Neomaskellia andropogonis Corbett , Trialeurodes ricini (Misra) , Aleuroclava pentatuberculata (Sundarraj & David) . Aphidoidea: Acyrthosiphon pisum (Harris) , Adelges sp. , Aphis affinis Del Guercio , Aphis craccivora Koch , Aphis fabae Scopoli , Aphis gossypii Glover , Aphis nerii Boyer de Fonscolombe , Asiphonella cynodonti (Das) , Brachycaudus pruni (Koch) , Dactynotus carthami (Hille Ris Lambers) , Uroleucon compositae (Theobald) , Hyalopterus atriplicis (Linnaeus) , Hyadaphis coriandri (Das) , Lipaphis pseudobrassicae (Kaltenbach) (as L. erysimi (Kaltenbach)) , Myzus persicae (Sulzer) , Rhopalosiphum nymphaeae (Linnaeus) , Therioaphis trifolii (Monell) , Aphis (Toxoptera) aurantii Boyer de Fonscolombe. Cicadellidae : Empoasca kerri Singh-Pruthi , Hishimonus phycitis (Distant) , Nephotettix virescens (Distant) , Orosius albicinctus Distant , groundnut jassids. Coccoidea: Coccidohystrix insolita (Green) , Comstockaspis perniciosa (Comstock) (= Quadraspidiotus perniciosus (Comstock)) , Ferrisia virgata (Cockerell) , Phenacoccus sp. , Pseudococcus cryptus Hempel (as P. citriculus Green ), Pseudococcus saccharicola Takahashi , Pseudococcus sp. , Maconellicoccus hirsutus (Green) , Gannaspis glomerata (Green) , Nipaecoccus viridis (Newstead) , Trabutina (as Naiacoccus ) sp. Delphacidae : Nilaparvata lugens (Stål) , Sogatella furcifera (Horvath) . Lophopidae : Pyrilla perpusilla (Walker) , Pyrilla spp. Psyllidae : Diaphorina citri Kuwayama , Psylla isitis Cotes. Lepidoptera : Crambidae : Chilo partellus (Swinhoe) . Pyralidae : eggs of Scirpophaga excerptalis (Walker) . Noctuidae : Earias vittella (Fabricius) , E. insulana (Boisduval) , early instar larvae of Helicoverpa armigera (Hübner) . Diptera : Anthomyiidae : Atherigona soccata Rondani. Acari : Tetranychidae : Oligonychus coffeae (Nietner) (as Tetranychus bioculatus Wood-Mason ), Tetranychus urticae Koch (as T. neocaledonicus Andre ). Feeds on pollen of Echinocloa colona on rice field bunds (Shanker et al. 2018).
Collected on a wide range of host plants including sugarcane, maize, brinjal, rice, wheat, cotton, bhendi, cowpea, groundnut, sunflower, safflower, sesamum, coconut, pigeonpea, castor, sorghum, cabbage, indigo, peach, cumin, mustard, lucerne, tobacco, sandal, pongamia, crotalaria, rice bean, coriander, soyabean, bittergourd, Japanese mint, Citrus limon, Euryale ferox , Thevetia neriifolia , Triumfetta s p., Achyranthes aspera , Tribulus terrestris , Abutilon indicum , Parthenium hysterophorus, Lathyrus sativus, and Sida spinosa (label data).
Seasonal occurrence. It is active throughout the year, except extreme winter ( Chandrababu et al. 1997a). Abundant on brinjal mealybug infestations almost throughout the year ( Puttarudriah & Channabasavanna 1957). It can be mass produced in the laboratory on Ferrisia virgata on potato sprouts.
Natural enemies. Larvae ( Fig. 21a View FIGURE 21 ) and pupae are parasitized by encyrtid and chalcid parasitoids such as Homalotylus hemipterinus De Stefani (Dalman) ( Hymenoptera : Encyrtidae ) ( Fig. 21b View FIGURE 21 ) and Lasiochalcidia sp. ( Hymenoptera : Chalcididae ) ( Fig. 21c View FIGURE 21 ).
Notes. Crotch (1874: 21) listed Brumus daldorfii Crotch as a replacement name for Coccinella suturalis Fabricius, 1798 as it was supposed to be preoccupied in Olivier (1791) ( Coccinella suturalis Olivier, 1791: 50 , from “Indes orientales”). The combination Brumoides daldorfii (Crotch) has not been used in any publication except Kovář’s Palaearctic checklist (2007) and Dorji et al.’s (2019) checklist of Coccinellidae of Bhutan. It is unclear why this name has not been used in any major works. Brumoides suturalis remains the most popular and widely known name for this species and it is used here to avoid confusion.
It is one of the most well studied Chilocorini of the Indian region because it is commonly found in agroecosystems in association with several major pests. In north-eastern India, it is less common than B. lineatus and both coexist in northern states of India and in the north-eastern region, B. lineatus is more predominant.
Several publications are available on its biology, hosts, predatory potential, mass production and use in applied biological control. Some notable works are as follows: Stebbing 1903 (description of adult, brief notes on life history); Kapur 1939, 1942 (bionomics, description of immature stages with illustrations); Puttarudriah & Channabasavanna 1953, 1956 (brief notes on biology, hosts); Garg & Sethi 1984 (population dynamics, effect of insecticides); Chandrababu et al. 1999 (biology), 1996 (economics of production), 1997b (feeding potential), 1997c (toxicity of insecticides); Gautam 1990 (mass production technique). Poorani & Lalitha (2018), Rafi et al. (2005) and Hayat et al. (2014) provided bried diagnostic and biological details on B. suturalis .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Coccinellinae |
|
Tribe |
Chilocorini |
|
Genus |
Brumoides suturalis (Fabricius)
| POORANI, J. 2023 |
Brumoides daldorfii
| Dorji, C. & Loday, P. & Vorst, O. 2019: 503 |
| Kovar, I. 2007: 592 |
Brumoides kolhapurensis
| Poorani, J. 2005: 186 |
| Sathe, T. V. & Bhosale, Y. A. 2001: 28 |
Brumoides suturalis
| Poorani, J. 2002: 310 |
| Chapin, E. A. 1965: 237 |
Brumus daldorfii
| Crotch, G. R. 1874: 21 |
Brumus suturalis
| Korschefsky, R. 1932: 267 |
| Mulsant, E. 1850: 494 |
Coccinella suturalis
| Fabricius, J. C. 1798: 78 |