Tarentola gigas, (BOCAGE, 1875)
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2011.00768.x |
|
DOI |
https://doi.org/10.5281/zenodo.5479632 |
|
persistent identifier |
https://treatment.plazi.org/id/03C28793-7021-5B03-FC55-FE051E33FD83 |
|
treatment provided by |
Marcus |
|
scientific name |
Tarentola gigas |
| status |
|
TARENTOLA GIGAS ( BOCAGE, 1875)
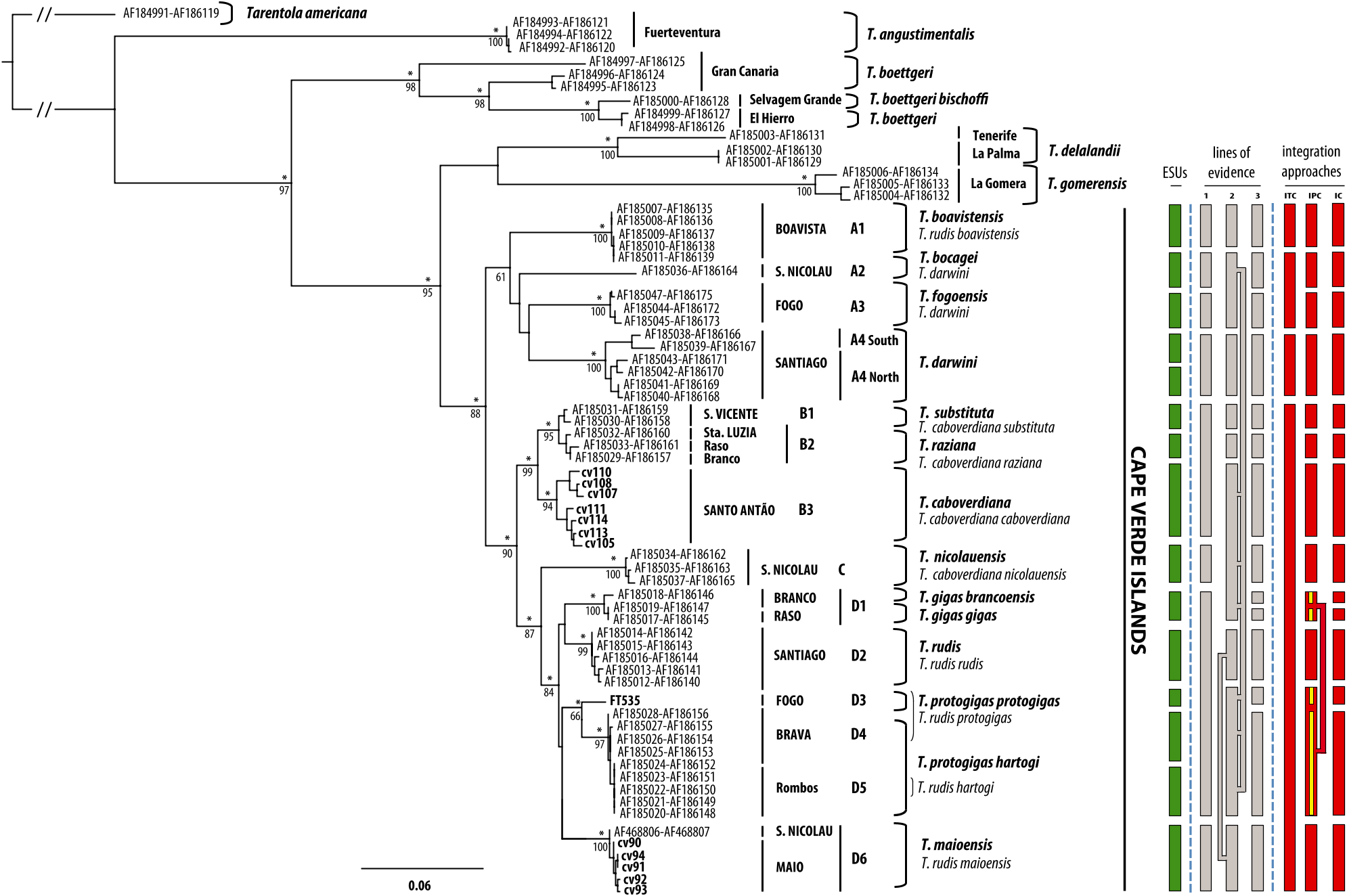
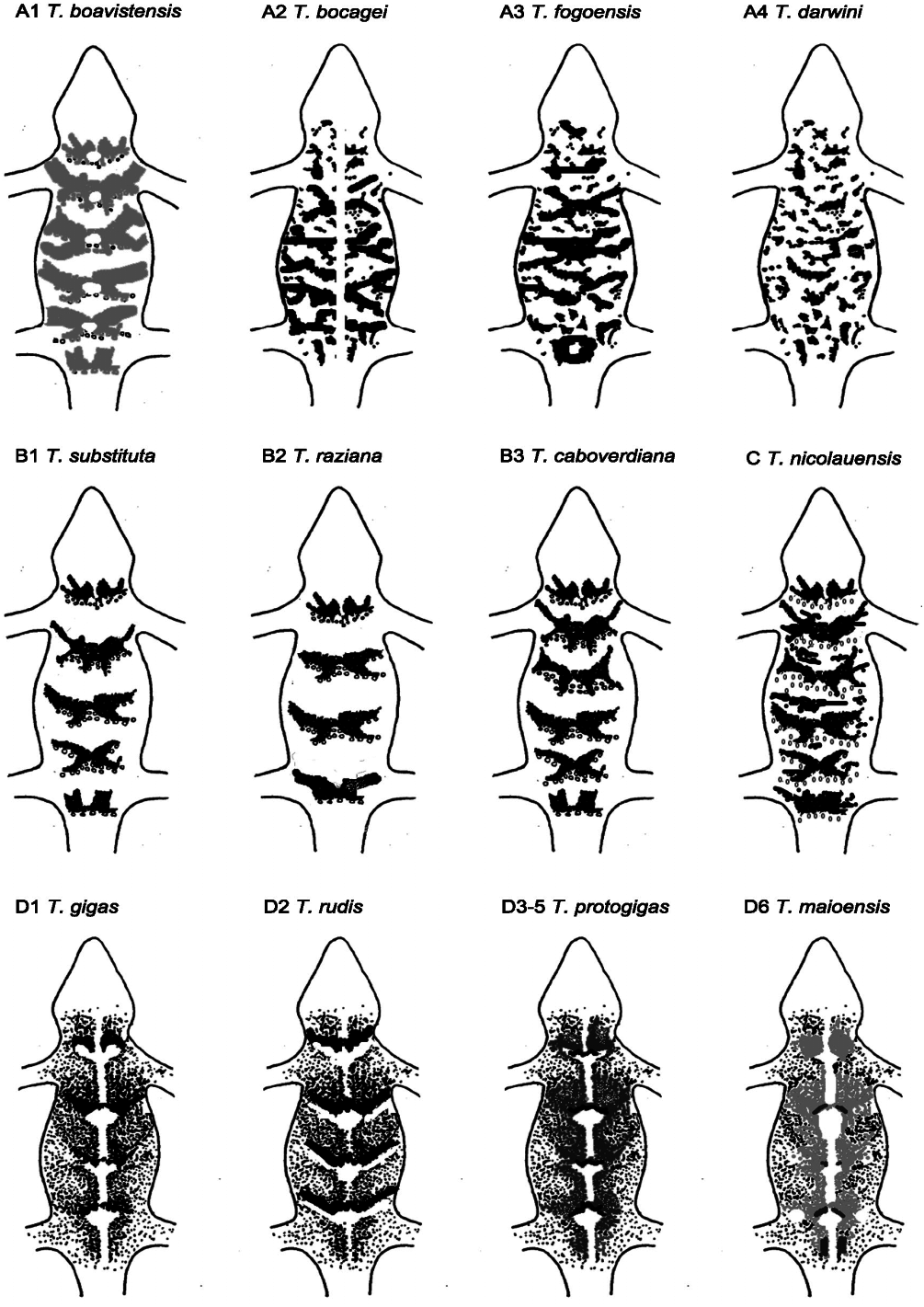
Diagnosis: Giant gecko with SVL above 100 mm [maximum SVL 155 mm ( Bocage, 1896), 103.6 mm on average; ( Schleich, 1987)]; eye/ear opening ratio 1.5– 2.0 ( Schleich, 1987); ear–eye/eye–snout distance ratio slightly ³ 1 ( Schleich, 1984, 1987). Eight to 12 supralabials and seven to nine infralabials ( Schleich, 1984); eight to 12 enlarged lamellae under the 4th finger; 160–195 midbody scales ( Joger, 1984b); flatter apical dorsal tubercles ( Fig. 5D View Figure 5 1 View Figure 1 ) with 16 transverse rows ( Schleich, 1984); several enlarged tubercles between the eye and the ear opening. Grey dorsal or olive greyish pattern with a broad, light well-defined middorsal line with generally five large saddle-like marks ( Figs 6D View Figure 6 1 View Figure 1 , 7D View Figure 7 1 View Figure 1 ); cream ventral parts, yellow on the lower parts; big dark spots on the labials, creating an alternating light and dark pattern; eye iris dark grey with a typical vertical light area around the pupil, joining the upper and lower parts of the eye which are also light.
It differs from other Tarentola from the same clade D, T. ‘ rudis ’ from Santiago, Fogo, Brava, Rombos, and Maio, besides from its size, by the absence of a keel on dorsal tubercles. Unlike all other Cape Verdean Tarentola , strong vocalisations play a clear role in social behaviour ( Schleich, 1982b, 1987). This species avoids vertical surfaces presumably due to its weight, and presents a robust body with typical extreme fat storage ( Schleich, 1987).
Distribution: Raso and Branco Islets, Cape Verde.
Genetic and phylogeographic remarks: Tarentola gigas is monophyletic in the mtDNA tree from Figure 2 View Figure 2 . Genetic divergence with other taxa within clade D is higher than among taxa within clade B, although lower than among members of clade A: D1–D2, D1–D3, D1–D4, D1–D5, and D1–D6 p- dist (cyt b) = 2.4 ± 0.8, 2.8 ± 0.9, 2.6 ± 0.9, 2.8 ± 0.9, and 3.9 ± 1.0%, respectively ( Table 5). Most of the Snn test values for PDC, ACM4, and MC1R are not significant among this clade (Appendix 5). According to the presently selected protocol of integration (IPC), a minimum of two lines of evidence differentiate T. gigas from all the other Tarentola from Cape Verde except T. protogigas from which it differs only in morphology ( Fig. 2 View Figure 2 ). Consequently, it is considered a different species, although not fulfilling the rule in respect to T. protogigas , due to several ecological, behavioural and geographical differences (see Discussion).
The two subspecies, T. g. gigas and T. g. brancoensis, are not reciprocally monophyletic ( Fig. 2 View Figure 2 ) and the level of genetic divergence is very low, p- dist (cyt b) = 0.2 ± 0.2% (data not shown). Only one of the three lines of evidence (morphology) differentiates the two island populations. Consequently, according to the IPC protocol, these are considered distinct subspecies ( Figs 2 View Figure 2 , 3 View Figure 3 and Appendix 3).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.