Bradypus variegatus, Schinz, 1825
|
publication ID |
https://doi.org/ 10.5281/zenodo.6621602 |
|
DOI |
https://doi.org/10.5281/zenodo.6621612 |
|
persistent identifier |
https://treatment.plazi.org/id/03C187E3-2B16-FFC0-66C8-F742FE10B913 |
|
treatment provided by |
Valdenar |
|
scientific name |
Bradypus variegatus |
| status |
|
1. View Plate 6
Brown-throated Three-toed Sloth
Bradypus variegatus View in CoL
French: Paresseux a gorge brune / German: Dreifinger-Faultier / Spanish: Perezoso tridactilo de garganta marrén
Other common names: Bolivian Three-toed Sloth, Brown-throated Sloth
Taxonomy. Bradypus variegatus Schinz, 1825 View in CoL ,
“Stidamerika.” Restricted by R. Mertens in 1925 to “Brasilien (wahr- scheinlich Bahia)” (= Brazil, probably
Bahia).
There have been up to nine subspecies recognized, but given their distribution, geographical variability, cryptic nature, and lack of clearly differentiated forms, the actual number is unclear, and subspecific taxonomyrequires reassessment.
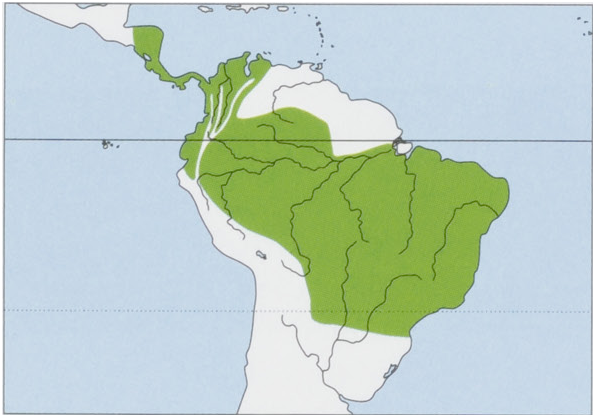
Distribution. From Honduras SE throughout Central America to Colombia and W & S Venezuela, and S into Ecuador, Peru, most of Brazil, N Bolivia, Paraguay, and N Argentina. View Figure
Descriptive notes. Head-body 519-540 mm, tail 52-55 mm, ear 8-22 mm, hindfoot 85-180 mm; weight 3.7-6 kg. Adult Brown-throated Three-toed Sloths weigh less than they appear due to long and thick pelage. Males are slightly smaller (less than 10%) than females. They are gray, with long, coarse hair, but hair is often covered with mutualistic algae (and other microbiota), causing it to have green tinge. The Brown-throated Three-toed Sloth has a dark orbital stripe over a lighter colored and browner face, giving the appearance of a mask. Males have dorsal speculum of shorter cream to orange colored hair, with dark stripe running vertically down the center of it. Contrasting dorsalstripe appears to become accentuated with age and is sometimes accompanied by black spots on either side. Males and females have small vestigial tails. Forelimbs and hindlimbs have three large and distinctive claws. The Brown-throated Three-toed Sloth has long forelimbs (370-450 mm) and relatively short hindlimbs (320-370 mm). Chromosomal complement is 2n = 54-55 and FN = 56-58.
Habitat. Tropical forests with diversity oftree species and complex horizontal andvertical cover. The Brown-throated Three-toed Sloth can reach high densities in agricultural landscapes if overhead canopy trees are maintained. It is a generalist and uses manydifferent tree species, although individuals will specialize on a few species of trees and even spend a disproportionate amount of time resting and foraging within a few individual trees, or “modal” trees, in their home ranges. In an agricultural landscape in Costa Rica, Brown-throated Three-toed Sloths were strongly dependent on intact tropical forest and two species oftrees in the nettle family (Cecropra obtusifolia and Coussapoa villosa, Urticaceae ). In general, trees that have crowns exposed to sunlight and those that contain masses oflianas are selected.
Food and Feeding. The Brown-throated Three-toed Sloth is a strict folivore, consuming leaves in canopies oftrees. Individuals typically consume leaves in trees where they spend a considerable amount oftime. Diets vary spatially and overall include leaves from more than 50 plant species. They prefer and mostly consume young leaves, presumably because of their nutritional value. The Brown-throated Three-toed Sloth has one of the slowest rates ofdigestion. Nevertheless, estimated leaf removal rate is 5-1 g ofleaves/kg/day. Sloths, in general, are foregut-fermenting mammals, and the Brownthroated Three-toed Sloth possess a unique gut community dominated bythe bacterial phyla Proteobacteria and Firmicutes. Three-toed sloths possess a highly conserved, low-diversity foregut community, with highly abundant species of Neisseria bacteria.
Breeding. The Brown-throated Three-toed Sloth is polygynous, with males excluding male competitors from their core home ranges. In one study in Costa Rica, only 25% of resident adult males sired offspring, and one individual male sired 50% of all juveniles. Sedentary lifestyle of the Brown-throated Three-toed Sloth appears to facilitate polygyny because multiple females can persist within small patches of habitat and be monopolized by a single male. Females appear to use important mating strategies by switching mates among breeding seasons and shifting their home ranges during estrus. The Brown-throated Three-toed Sloth breeds seasonally. Gestation is ¢.6 months in length, and females give birth to one young over a six-month period that peaks in late February. Neonates are dependent on their mothers, clinging to their venters for 100 days or more. This relatively short time to offspring independence allows females to breed in most years; on average, nearly 75%ofadult females produce young in the wild, and this frequency appears to vary little from year-to-year. Juvenile survival can be high but is lowest immediately following maternal independence.
Activity patterns. Brown-throated Three-toed Sloths are active diurnally and nocturnally, but they are more often active during daylight hours and generally avoid activity during the crepuscular periods of the day. Adults usually sleep for 15-18 hours/day, either holding on to a tree with their claws or resting in the crotch of a tree with their heads tucked into their chests. Three-toed sloths have a high degree of heterothermy in their body temperature, and therefore, they behaviorally adjust to ambient temperatures throughout the day. Individuals can be observed in the mornings ascending to the top of the canopy, presumably to warm in full sunlight, and descending into shade as daytime temperatures increase. During rainstorms, Brown-throated Threetoed Sloths do not seek shelter but typically rest in place.
Movements, Home range and Social organization. Brown-throated Three-toed Sloths climb slow and deliberately and position themselves on undersides of branches with their bodies perpendicular to branches when moving. In a single day, an individual only moves an average of 40 m and is often found on the same tree. Once a week, individuals descend to the forest floor. While on the ground, they create shallow depressions with their vestigial tails and defecate. This ritualized behavior reinforces sloth moth ( Pyralidae ) colonization of fur and increases nitrogen and phosphorous levels in sloth fur contributing to algal growth. Home range sizes do not differ between sexes or by breeding status and are 0-1-19 ha. Most adult male Brown-throated Three-toed Sloths maintain exclusive use of core areas in their home ranges. Home ranges of males overlap with many females but not each other, and home ranges of adult females overlap each other. Juveniles strongly selectfor forest cover during dispersal, avoiding agricultural habitat types.
Status and Conservation. CITES Appendix II. Classified as Least Concern on The [UCN Red List. The Brown-throated Three-toed Sloth has the widest distribution of the four species of Bradypus and occurs across various forest types. It occurs in protected areas and is rarely targeted for consumption or other human uses, although occasionally it is hunted in Central America and South America by indigenous groups and for the pet trade. Clearing of tropical forests for crops grown in monoculturesis particularly detrimental because these plantations retain little forest structure. Brown-throated Threetoed Sloths are more vulnerable to habitat loss and fragmentation than other sloths, especially two-toed sloths ( Choloepus spp. ). Nevertheless, previous work has shown that shade-grown agriculture, like that found in certain types of cacao plantations embedded within an agroecosystem, can support viable populations of the Brown-throated Three-toed Sloth, if immigration from surrounding forests is maintained. In particular, intact riparian forests provide critical movement corridors for maintaining connectivity of populations in modified tropical landscapes.
Bibliography. Dill-McFarland et al. (2016), Garcés-Restrepo et al. (2017), Hayssen (2010), Mendoza etal. (2015), Mertens (1925), Montgomery & Sunquist (1978), Moraes-Barros etal. (2010), Noss et al. (2008), Pauli & Peery (2012), Pauli, Mendoza et al. (2014), Pauli, Peery et al. (2016), Peery & Pauli (2014), Sunquist & Montgomery (1973b), Svartman (2012).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.